Arsenic exposure, metabolism, and health effects
Arsenic is known as a poison and human carcinogen. Arsenic trioxide (As2O3), a water-soluble powder that produces a colorless, tasteless, and odorless solution, was a favorite homicidal agent during the Middle Ages and its use continues today, although not to the same extent. There is much debate on Napoleon's death, whether he was poisoned by arsenic-tainted wine during his exile on the island of St. Helena.
Arsenic is a trace element found in the earth’s crust at an average concentration of ~5 µg/g (ppm). Although its relative abundance in the earth’s crust is about 54th, arsenic can become concentrated in some parts of the world because of natural mineralization. Arsenic is a component of 245 minerals, associated most frequently with other metals such as copper, gold, lead, and zinc in sulfidic ores. When disturbed by natural processes, such as weathering, biological activity, and volcanic eruption, arsenic may be released into the environment. Anthropogenic activities, such as combustion of fossil fuel, mining, ore smelting, and well drilling, also mobilize and introduce arsenic into the environment.
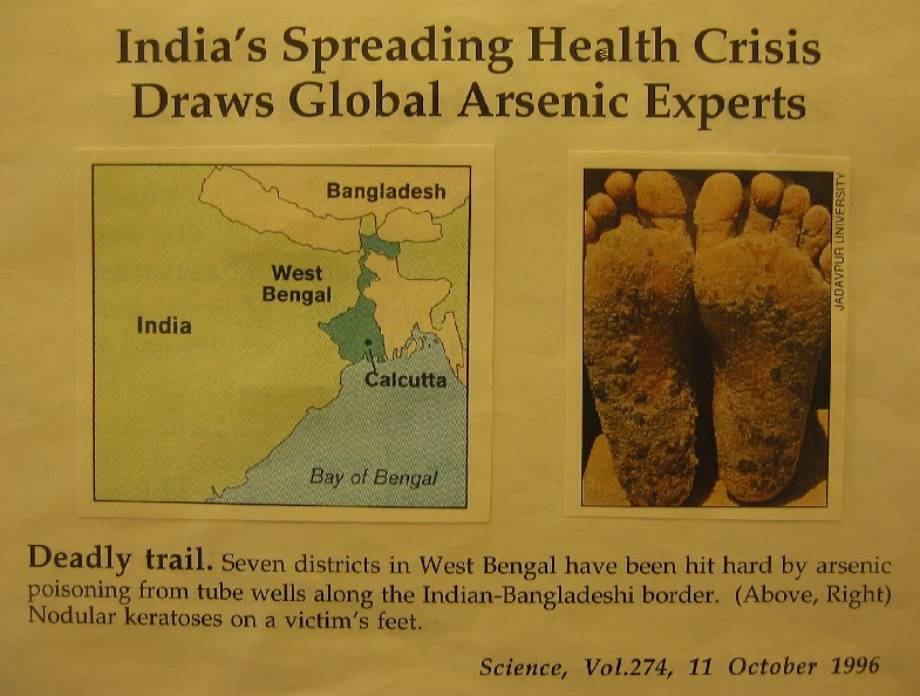
The devastating arsenic problem is making history as the cause of the largest mass poisoning ever. In Bangladesh and India alone, millions of people are affected by high levels of arsenic present in drinking water. Millions of wells have been installed since the 1970's with the intention of providing clean water, free of microbial pathogens. However, the bedrock containing naturally high levels of arsenic releases arsenic into the water, resulting in water arsenic concentration as high as several hundred micrograms per liter (µg/L) or even milligrams per liter (mg/L) in some wells. Ingestion of such high levels of arsenic in drinking water over several years can lead to a variety of adverse health effects including cancers of the skin, bladder, and lung as well as possible neurological and cardiovascular effects.
The magnitude of the arsenic problem and the seriousness of the adverse health outcomes resulting from exposure to arsenic make arsenic the single most important environmental contaminant. As a consequence, regulations on arsenic have become more stringent.

The major arsenic species in shrimp is the innocuous arsenobetaine
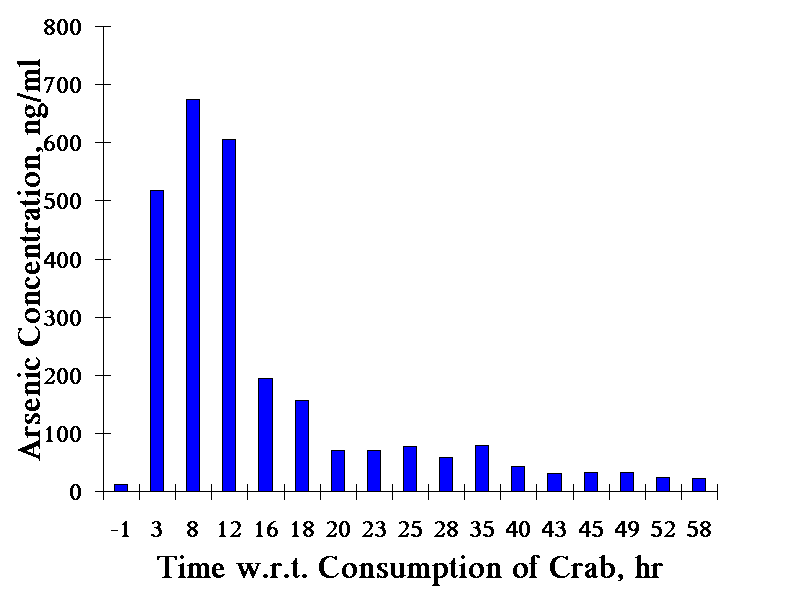
Arsenic is also abundant in seafood at concentrations as high as several hundred micrograms per gram (µg/g). Arsenobetaine is the major arsenic species in most seafood (crustaceans). It is excreted into urine without metabolism, and is essentially non-toxic.

Arsenosugars are abundant in seaweed, mussels, oysters, and clams. They are metabolized in the body to several new arsenic species.
Arsenobetaine (AsB) is excreted unmodified.
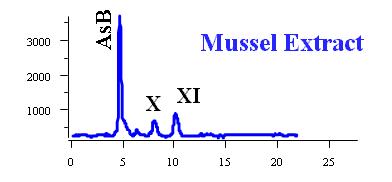
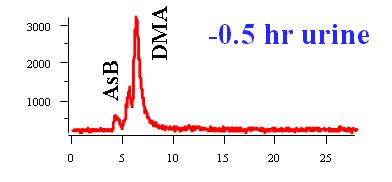
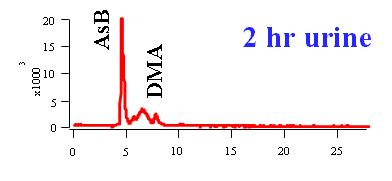
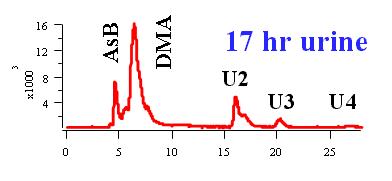
More than 100 arsenic compounds (species) are present in the natural environment and biological systems. The following Table lists some of the common arsenic species. Although the trivalent arsenic species, such as inorganic arsenite (AsIII), monomethylarsonous acid (MMAIII), and dimethylarsinous acid (DMAIII) are highly toxic (8-13), arsenobetaine (AsB), the predominant arsenic species present in most crustaceans, is essentially non-toxic.
| Example of arsenic species present in biological systems | ||
|---|---|---|
| Name | Abbreviation | Chemical formula |
| Arsenite, Arsenous acid | AsIII | As(OH)3 |
| Arsenate, Arsenic acid | AsV | AsO(OH)3 |
| Monomethylarsonic acid | MMAV | CH3AsO(OH)2 |
| Monomethylarsonous acid | MMAIII | CH3As(OH)2 |
| Dimethylarsinic acid | DMAV | (CH3)2AsO(OH) |
| Dimethylarsinous acid | DMAIII | (CH3)2AsOH |
| Trimethylarsine oxide | TMAO | (CH3)3AsO |
| Trimethylarsine | TMAIII | (CH3)3As |
| Arsenobetaine | AsB | (CH3)3As+CH2COO- |
| Arsenochline | AsC | (CH3)3As+CH2CH2OH |
| Tetramethylarsonium ion | Me4As+ | (CHM3)4As+ |
| Dimethylarsinoyl ethanol | DMAE | (CHM3)2AsOCH2CH2OH |
| Arsenosugars | X, XI, XII, XIII, XIV, XV | See structure below |
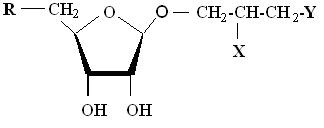
| R | X | Y | |
|---|---|---|---|
| X | (CH3)2As(O)- | -OH | -OH |
| XI | (CH3)2As(O)- | -OH | -OPO |
| XII | (CH3)2As(O)- | -OH | -SO3H |
| XIII | (CH3)2As(O)- | -OH | -OSO3H |
| XIV | (CH3)2As(O)- | -NH2 | -SO3H |
| XV | (CH3)2As+- | -OH | -OSO3H |
Research on arsenic in this group covers a broad range, from the development of arsenic speciation techniques to the application of these techniques to the understanding of arsenic biogeochemical cycling, metabolism, and toxicological effects. A Collaboration project also involves the use of arsenic compounds for treating acute promyelocytic leukemia (APL).
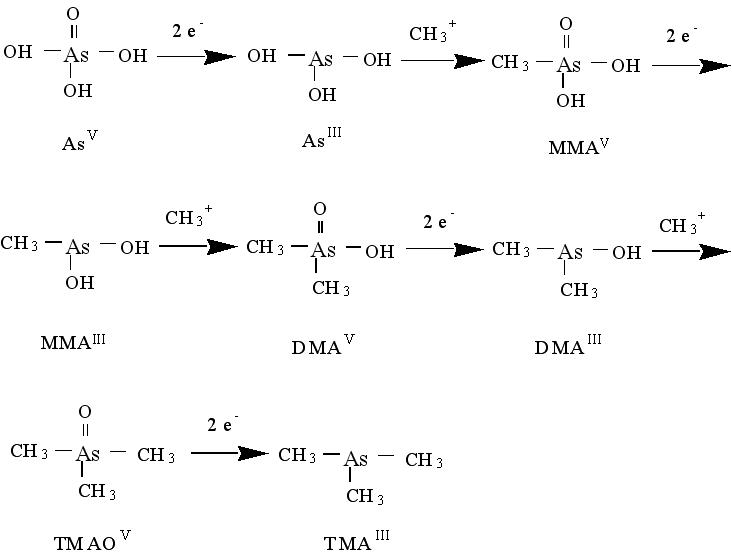
Biomethylation is the main mechanism for the metabolism of inorganic arsenic. It involves alternate steps of two-electron reduction followed by oxidative addition of a methyl group.
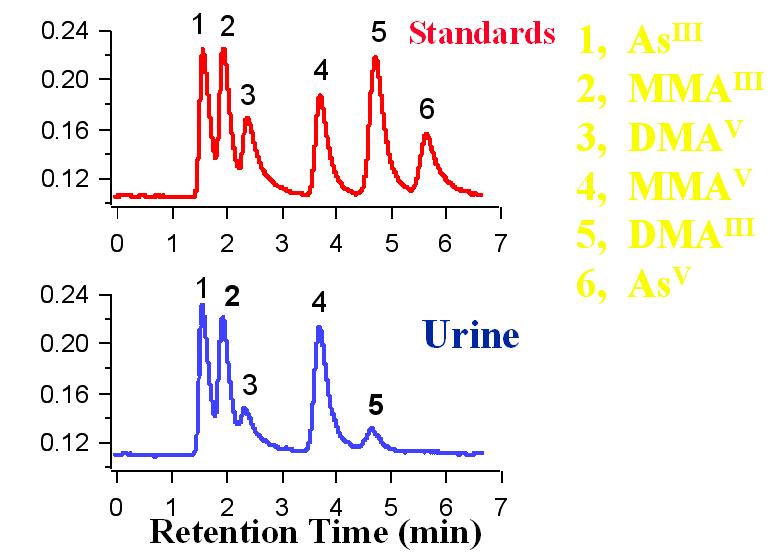
Speciation of arsenate (AsV), arsenite (AsIII), monomethylarsonic acid (MMAV), monomethylarsonous acid (MMAIII), dimethylarsinic acid (DMAV), and dimethylarsinous acid (DMAIII). The arsenic species were separated using ion pair chromatography and were detected with hydride generation atomic fluorescence spectometry.
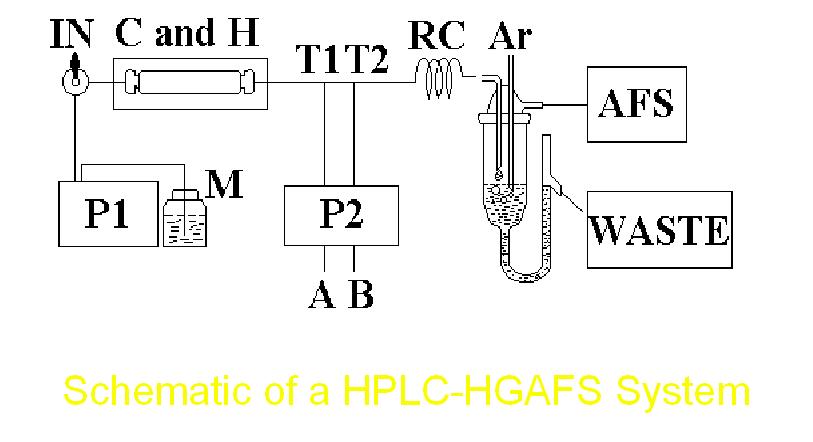
A typical set-up of HPLC-HGAFS
MMAIII binding with metallothionein (MT) demonstrates an example of mass spectrometry studies of interactions between trivalent arsenicals and proteins. Electrospray ionization with tandem mass spectrometry provides information on binding stoichiometry.
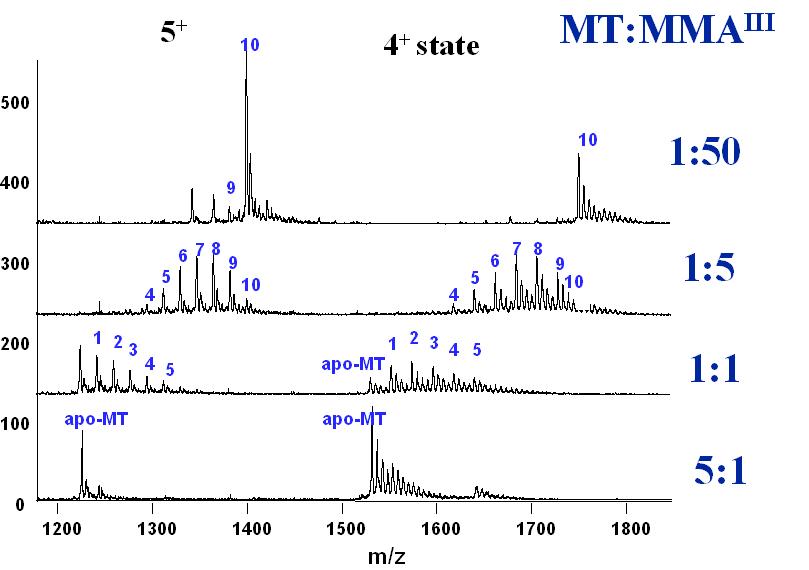
Mass spectra of MMAIII binding with metallothionein

A hybrid quadrupole time-of-flight mass spectrometer
