Affinity Capillary Electrophoresis with Laser-Induced Fluorescence Polarization Detection
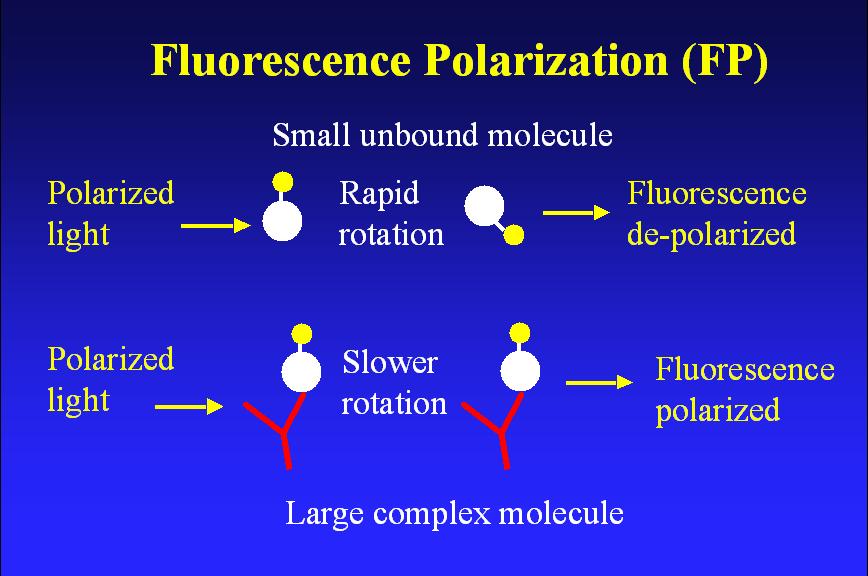
Schematic illustrating fluorescence polarization
Capillary electrophoresis (CE) combined with molecular recognition is suitable for bioanalytical applications involving a wide variety of biomolecules. Previous studies of binding interactions using CE involve multiple-step titration experiments and are time consuming. We are developing new approaches based on laser induced fluorescence polarization (LIFP) detection for CE separation, which allows for on-line monitoring of affinity complex formation.

A lab-built CE-LIPF system
Most fluorescence measurements for CE detection have been based on the fluorescence intensity. An important fluorescence characteristic, fluorescence polarization, has not been extensively explored for CE detection. Fluorescence polarization is a measure of the time-averaged rotational motion of fluorescent molecules. A fluorescent molecule, when excited by a polarized light, will emit fluorescence with its polarization primarily determined by the rotational motion of the molecule. Since the molecular rotation is inversely proportional to the molecular volume, the polarization is in turn related to the molecular size. A small molecule rotates fast in solution and exhibits low value of polarization whereas a large molecule exhibits a higher polarization because of its slower motion under the same conditions. Thus, changes in fluorescence polarization can reflect the association or dissociation between molecules of interest. This is a very useful feature for affinity CE studies where affinity complexes are formed prior to or during CE separation. We have developed a CE-LIFP system and demonstrated its applications in studies of common binding systems representing peptide-protein, protein-protein, and DNA-protein interactions.
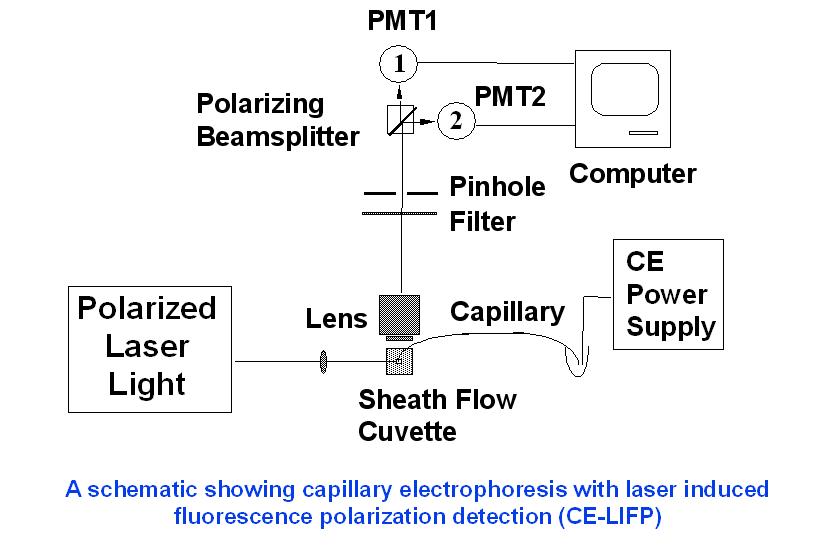
Schematic diagram showing CE-LIPF
