OUR APPROACHES
In our laboratory, we use a multi-disciplinary approach to explore brain function and dysfunction in vitro and in vivo. With a primary interest in bran damage and recovery after stroke and the pathophysiology of the brain that leads to cognitive impairment in disorders such as schizophrenia, we attempt to identify novel patterns of adaptive or maladaptive change in the brain then identify the processes that lead to these changes. We incorporate in vivo imaging to optically record the structure and functional with mesoscopic (regional) and microscopic (cellular and subcellular) resolution.
We combine our imaging approaches with behavioural assessment, quantitative immunoassays, and studies in cell culture to validate our imaging findings and provide mechansistic insight. Long term, our goal is to identify new and better therapies to prevent or treat cognitive or sensorimotor disabilities associated with brain disorders.
Our research projects are described in more detail in the Research section. A major research interest over the last several years has been the study of collateral blood flow during stroke. Collaterals are vascular redundancies in the brain that allow transfer of blood between different arterial systems in the brain, and we are both characterizing the changes in blood flow that occur during an ischemic stroke and investigating therapeutics to augment collateral flow. Collateral therapeutics may lead to improved acute stroke treatment, but most survivors of stroke are still left with some disability. For this reason, we are investigating the plasticity of the nervous system after stroke and methods to augment the "rewiring" process that underlies recovery from brain damage. A related project examines the precise functional organization of the sensorimotor cortex that allows for the coding of different somatosensory stimuli, and the plasticity of this system after brain injury after stroke. Finally, we are interested in how specialized structures in the central nervous system called perineuronal nets contribute to normal and abnormal brain activity, and how the loss of these structures may lead to cognitive impairment in disorders such as schizophrenia.
Within these projects, we use a variety of tools to measure brain and behaviour. A description of some of these approaches can be found below.
Blood Flow Multi-Photon Microscopy
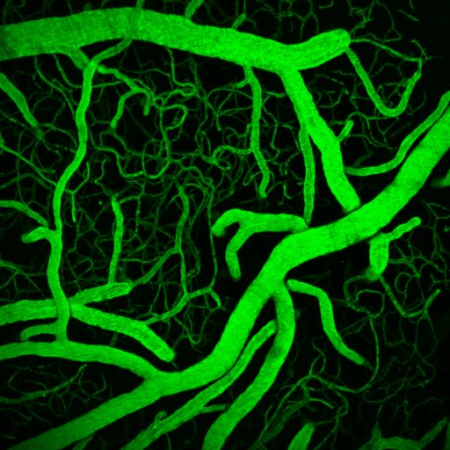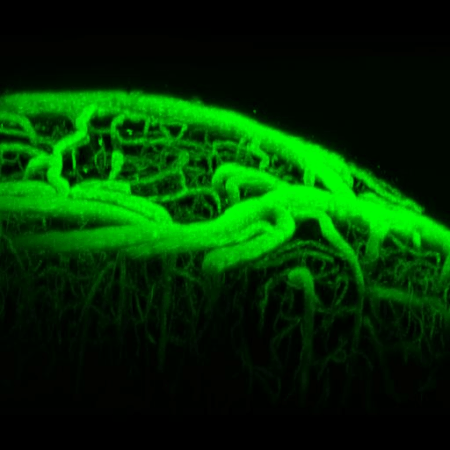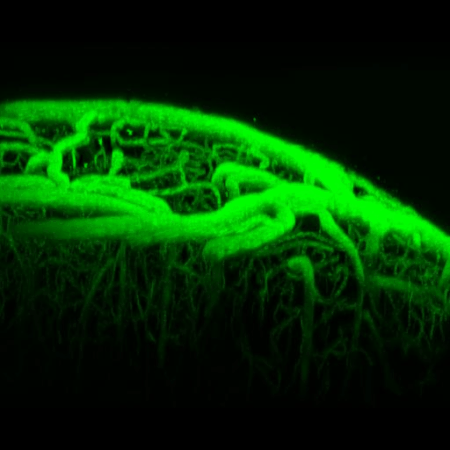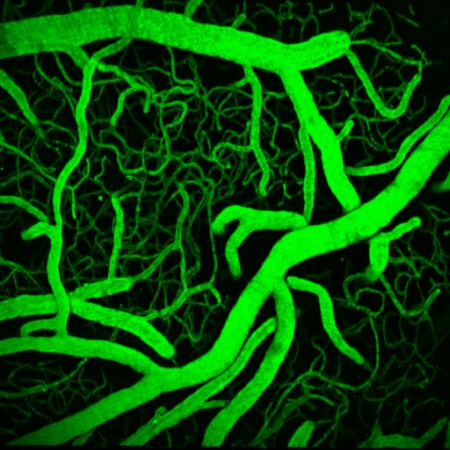
Multiphoton or two-photon laser scanning microscopy is an approach to confocal microscopy that allows deep penetration into opaque tissues such as the brain. Using special lasers to "optically section" the cortex, we can image deeper into cortex to study brain structure, function, or blood flow.
Our lab is home to a cutting edge Leica SP5 confocal and multiphoton microscopy system with multiple visible laser lines and two infrared pulse lasers for in vivo imaging and optogenetics. To image blood flow, we can label the blood plasma with fluorophores, then perform multiphoton microscopy to measure fluorescence in the vessel lumen. We use this approach to create high resolution three dimensional maps of cortical vessels (including collaterals) after stroke and measure the vasoactive properties of different treatments. We can also use multiphoton microscopy to measure the exact speed and direction of red blood cell movement in arterioles, veins, and capillaries of the brain.
Multi-Photon Calcium Imaging
By expressing genetically encoded calcium indicators in different classes of neurons in the brain, we can monitor neuronal firing in intact local networks. When neurons fire action potentials, calcium in the soma and processes increases, and calcium indicators reflect this change by emitting more fluorescence.
Using transgenic mice and vectors to express different indicators and fluorophores, we can image spontaneous or sensory evoked activity in hundreds of neurons simulataneously. Using other genetically encoded indicators or calcium dyes, we can also detect calcium signals in astrocytes. The image at left shows neurons (green) and astrocytes (orange) in the mouse cortex labeled with a calcium indicator to detect neuronal spiking or astrocyte calcium transients (as well as sulforhodamine 101 to label astrocytes in red). Different cells are visible as we focus deeper into the cortex.
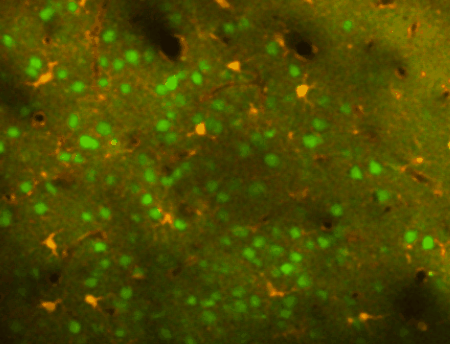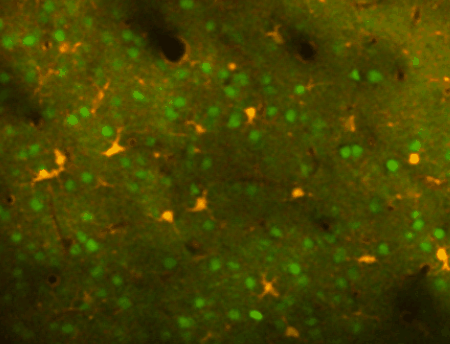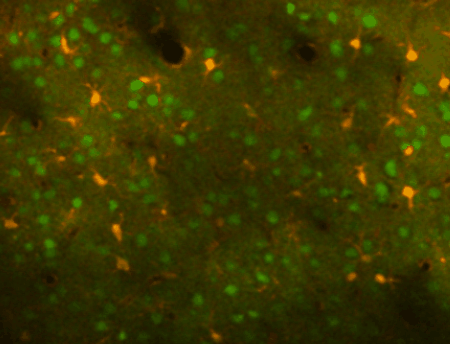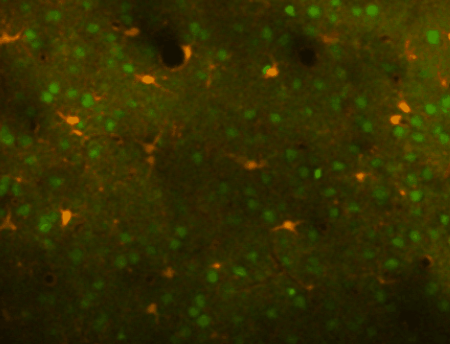
In-vivo regional (mesoscale) imaging
We use a variety of regional imaging approaches to map regions of activity or regional blood flow in the intact brain. Intrinsic signal imaging and flavoprotein autofluorescence imaging are used in the lab to map regions of cortex responsible for processing sensory information from different limbs. We use these tools to map how these regions change after brain injury such as stroke.
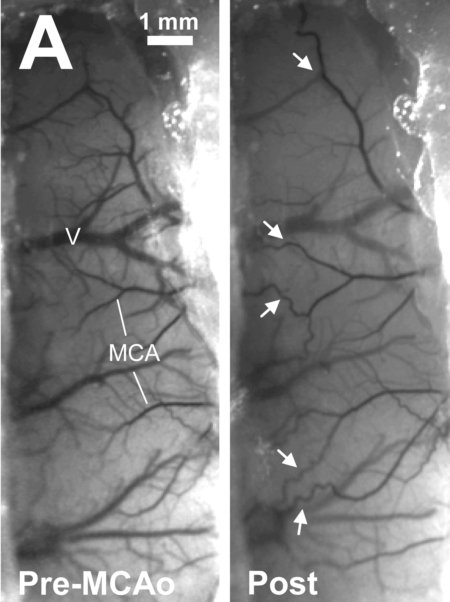
Laser speckle contrast imaging (shown at left) allows us to map blood flow (darker vessels reflect faster flow) in the cortex with resolution to individual vessels. The image at left shows the formation of collateral connections (white arrows) between different arteries after onset of a stroke.
Spectral COnfocal REflectance Microscopy
Our lab uses a technique called SCoRe (Spectral COnfocal REflectance microscopy) to allow for label free imaging of myelinated fibres in the intact brain. Myelin is critical to signal transmission of neurons and brain function as a whole. After stroke, myelin can be damaged and transmission disturbed.
Our lab is investigating the timeline of myelination changes after stroke utilizing SCORE imaging. In addition to this in vivo confocal approach, our SP5 has four internal spectral detectors and a full suite of visible light laser lines for confocal microscopy of cells in culture or tissue on slides. We use these approaches to image immunofluorescent markers and to map neuroanatomical changes associated with brain damage and recovery in sectioned brain and spinal tissue.

Epifluorescent Microscopy
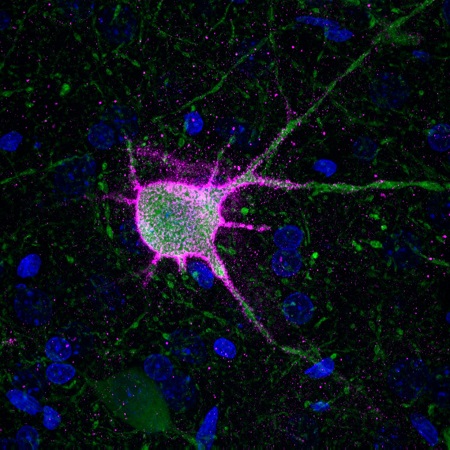
Standard immunofluorescent imaging is used to image fluorescent markers in slide mounted brain and spinal tissue. An image of a parvalbumin neuron (green) and its perineuronal net (pink, DAPI nucleic stain in blue) collected on our upright Leica DM6000 microscope are shown at left.
Behavioural Assessments
We use a variety of approaches to measure the sensorimotor or cognitive function of rodents. Most commonly, we use sensitive sensorimotor tests (such as single pellet skilled reaching, shown at left) to measure the recovery of motor functions after stroke and treatment.
Rodents have reaching movements very comparable to humans, and their recovery of function after a stroke parallels human recovery from stroke induced sensorimotor impairment. We use these and other approaches to determine if our novel treatments are beneficial for recovery.

Cell Culture
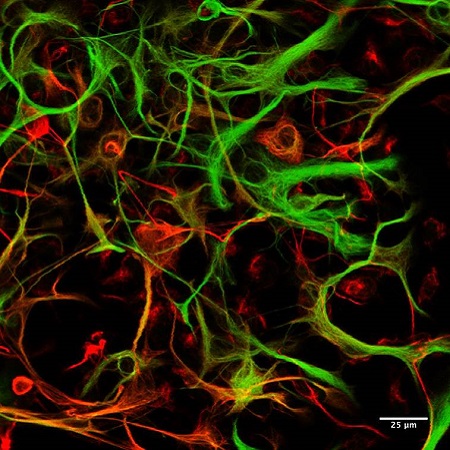
While most of our work is in vivo, in collaboration with Dr. Kathryn Todd's lab we often use isolated glial or neuronal culture to provide mechanistic insight into our research questions.
We have used isolated microglia (the brain's immune cells) cultures to study how the inflammatory activity of these cells differs between the brain and spinal cord. More recently, we have investigated the effect of novel pharmacotherapies to drive neuronal rewiring on neurons in culture and indirect effects on glial cells.
Other Techniques
As part of the Neurochemical Research Unit, our lab has access to a comprehensive suite of facilites for other standard laboratory techniques, including brain and spinal histology, quantitative immunoassays (Western blots, ELISAs), immunohistochemistry, and analytical neurochemistry.