RESEARCH IN THE WINSHIP LAB
Our Interests
In our laboratory, we use a multi-disciplinary approach to explore brain function and dysfunction in vitro and in vivo. We are currently attempting to better understand the failure of the collateral circulation during acute stroke that leads to poor outcome and develop new approaches to improve collateral flow during stroke ("collateral therapeutics"). Given that most stroke survivors are left with permanent disability, we are also exploring methods to restart recovery during chronic stroke by facilitating rewiring of the brain and spinal cord by modulating the inhibitory actions of chondroitin sulfate proteoglycans. Outside of stroke, we have new research investigating the role of perineuronal nets in the cognitive dysfunction associated with schizophrenia, and a strong interest in using advanced imaging to better elucidate the mechanisms of sensory encoding. More detailed descriptions of ongoing research in the lab can be found below.
Collateral Blood Flow and Stroke
Stroke refers to a sudden loss of brain function due to an interruption of flow of blood to the brain. Because stroke-induced brain damage evolves over days and weeks after stroke onset, early neuroprotective interventions can preserve brain tissue and improve outcome. Alternatively, delayed treatments that help the brain rebuild lost connections may have significant clinical value.
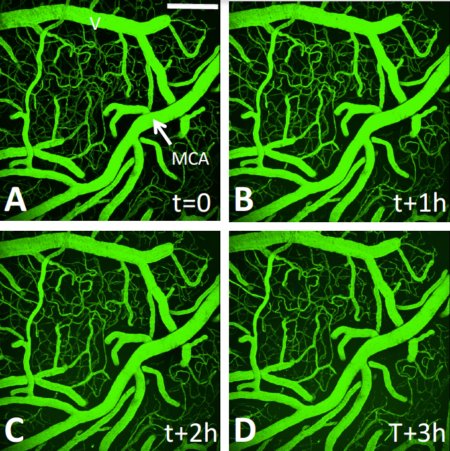
Our primary neuroprotective project involves evaluating strategies to improve blood flow to ischemic territories through collateral circulation. Collateral blood flow refers to subsidiary vascular networks that are recruited to restore blood flow to ischemic regions after stroke. It has been suggested that cerebral blood flow augmentation may increase blood flow to ischemic territories through these alternate collateral pathways. Using in vivo imaging techniques (see multiphoton time lapse of cerebral vasculature at left) that allow for precise mapping of blood flow in ischemic tissue, we evaluate the neuroprotective efficacy and degree of reperfusion associated with novel approaches to collateral blood flow augmentation. Given the high failure rate of clot busting, the importance of an effective alternate therapies cannot be overstated. Our goal is to develop new systemic treatments that can be easily and safely administered to patients that will increase collateral flow and keep tissue healthy until reperfusion. Supported by AIHS, CIHR, and QNRF.
Microcirculatory failure during stroke
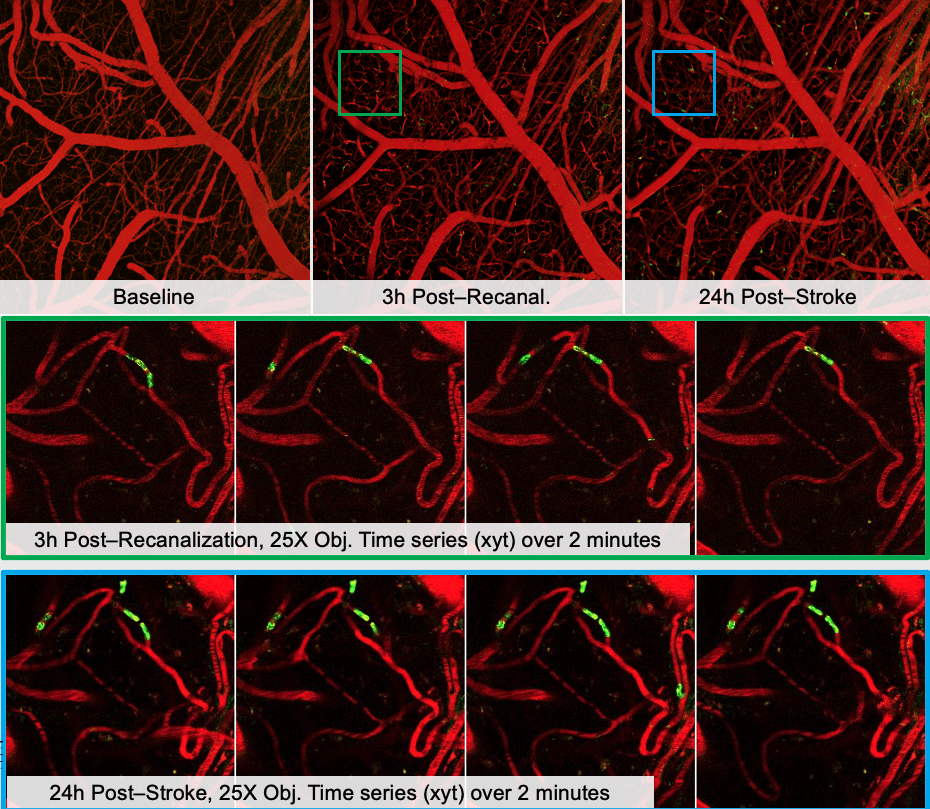
Recent studies have shown that after stroke, neutrophils can aggregate and occlude the capillaries, causing collateral downstream and worsen stroke outcomes. Reperfusion of the microvasculature is critical to improve stroke recovery. Endothelin-1 (ET-1) is a vasoconstrictor that can act on ET-1A receptors on neutrophils to promote their adhesion to the vessels.
We are interested in targetting ET-1 pharmacologically and examine its effects on neutrophil aggregation, and consequently, stroke outcomes. Supported by CIHR.
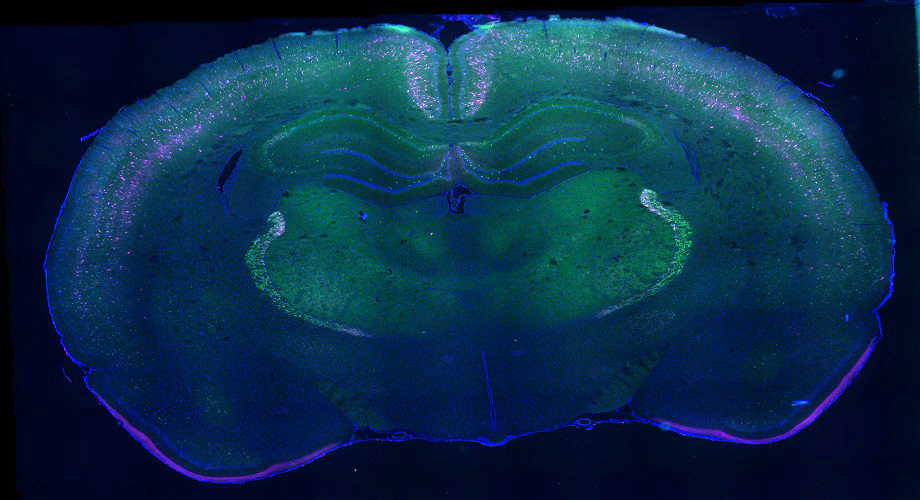
Plasticity and Recovery after Stroke
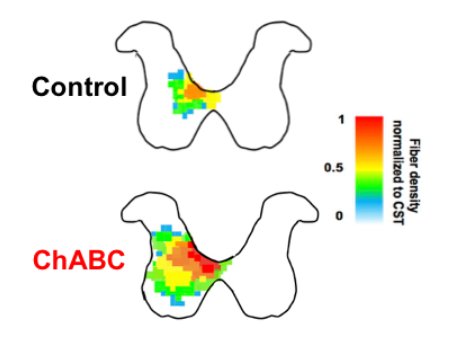
Stroke refers to a sudden loss of brain function due to an interruption of flow of blood to the brain. Because stroke-induced brain damage, recovery of function after stroke-induced cortical injury occurs through adaptive plasticity in surviving neurons that replaces the function of lost networks in the weeks and months following the initial insult. However, recovery is incomplete in large part due to growth inhibiting components in the central nervous system such as chondroitin sulfate proteoglycans (CSPGs). Given the potential for surviving brain tissue to reorganize and compensate for lost function, treatments that can counteract these inhibitory CSPGs and augment the brains endogenous repair mechanisms as an adjuvant to rehabilitation have considerable promise.
Our current projects use cell culture, molecular assays, in vivo imaging, and behavioural assessment to delve into plasticity of neuronal connectivity proximal and distal to the stroke. By defining adaptive changes in sensorimotor regions distal to the lesion, we hope to identify new opportunities for therapeutic intervention. Moreover, we are exploring several methods of degrading CSPGs (see increased innervation of spinal cord after stroke and intraspinal injection of ChABC at left), modulating their signaling, or preventing their formation either locally in the spinal cord or throughout the CNS as tools to augment recovery and rehabilitation. Supported by AIHS and the HSFC.
Sensorimotor Function and Plasticity
In our laboratory, we use advanced high resolution in vivo neuroimaging tools in animal models to provide insight into brain function and plasticity not possible via other methods. Our long term goals are to define the rules by which different classes of neurons regulate the spontaneous and evoked activity of their local neuronal network, and how these contributions change due to experience or injury.
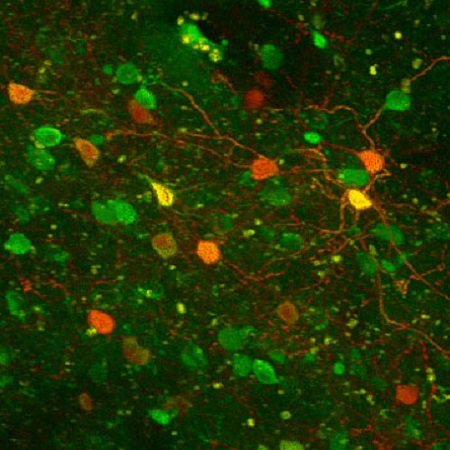
A current focus of our research is how different modalities of complex somatosensory information are encoded in the primary somatosensory cortex, and how the activity of parvalbumin neurons with or without an associated perineuronal net regulate local neuronal networks. Image at left shows neurons expresssing genetically endoded indicators in glutamatergic neurons (green) and parvalbumin inhibitory interneurons (orange). Supported by AIHS and NSERC.
Perineuronal Nets in Brain Function and Dysfunction
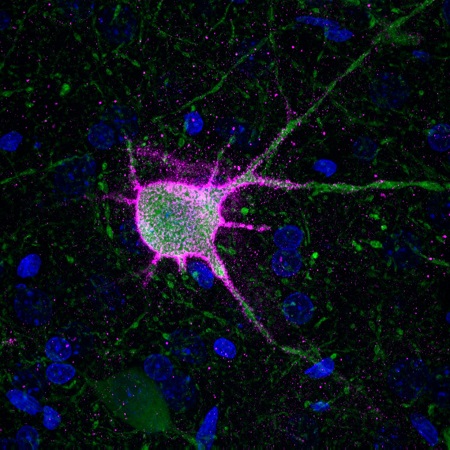
Perineuronal nets (see parvalbumin neuron (green) at left ensheathed by a PNN (pink)) are highly organized components of the extracellular matrix which surround mature neurons of the central nervous system. They are thought to restrict neuronal plasticity, or the capacity for neurons to change in structure or function. These structures are thought to be essential to the progression of critical periods of plasticity, within which a brain region undergoes dramatic reorganization. An example of this is in the visual cortex, where immature cells in infants rapidly mature into a functional visual cortex.
These structures have also been implicated in the development of schizophrenia. Perineuronal nets in the prefrontal cortex typically develop and mature during adolescence and early adulthood. Coincidentally, this is also when schizophrenia typically first manifests, and perineuronal nets have been shown to be reduced in the brains of patients suffering from schizophrenia. Our lab is investigating the role of perineuronal nets in schizophrenia using animal models of the disease. Supported by CIHR, NSERC, and the UHF.
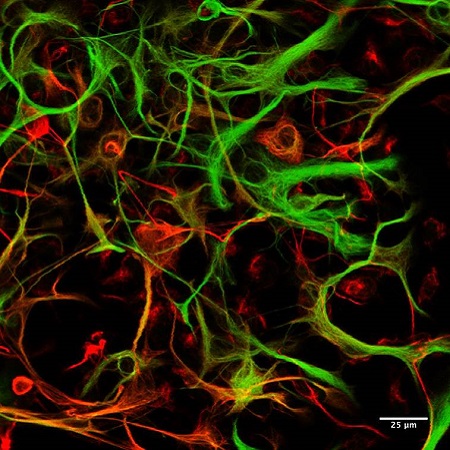
Neuronal and Glial Cells in Neuronal Plasticity
While most of our research is performed in vivo, we use in vitro cell culture preparations to provide mechanistic insight into functional relationships between neurons and glia. We are particularly interested in direct (neuronal) and indirect (via modulation of glial signaling or cytokine release) of treatments that modulate CSPGs or CSPG receptors. Using a combination of neuronal cell lines, primary neuronal cultures, isolated microglial cultures, and mixed glial cultures we are exploring the multifaceted effects of CSPG modulation on different classes of brain cells and the consequences for neuronal plasticity (i.e. neurite extension).