One of the most useful (and beautiful) facts about atoms is that every one is exactly like every one else of its kind in the universe. When we know the properties of one rubidium atom, for instance, we know about every other rubidium atom that exists.
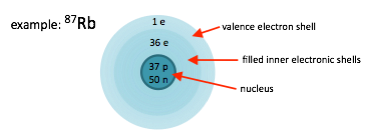
Why do I keep talking about rubidium? It’s one of the alkali atoms, the ones in the first column of the periodic table, and so it acts a lot like hydrogen, the simplest atom there is. The particular property we find useful is that these atoms have only one electron in the outermost shell, and we can therefore manipulate it with relative ease.
Because it’s one of the most popular atoms for laser cooling and trapping, let’s consider rubidium. The isotope with 87 nucleons has particular nice properties. It has 37 protons and 50 neutrons in the nucleus, 36 electrons in the inner electronic shells, and one valence electron.
Manipulation of the internal state of the atom is easiest through the unpaired valence electron. We can use various kinds of electromagnetic fields to manipulate the different degrees of freedom of this atom, each at a different energy scale.
