Fuel Cell Research
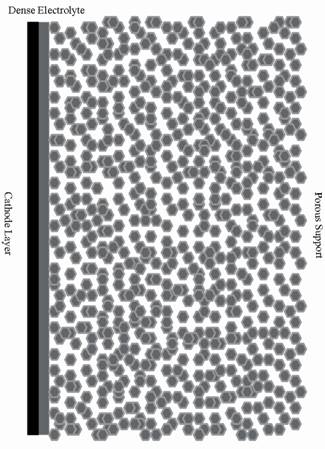
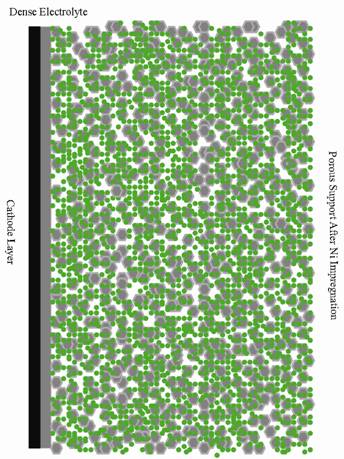
Development of a New Generation of Tubular SOFCs in University of Alberta Ceramic fuel cells convert the chemical energy of a fuel to electrical power. The main advantages of this type of fuel cell are their high efficiency, fuel flexibility and minimal contribution to pollution of the environment. The first generation of tubular solid oxide fuel cells (SOFCs) which had low power density was developed in the early 1990's [1]. They should be operated at high temperatures in order to obtain sufficient power which then leads to electrode sintering, interfacial diffusion, cracking due to thermal mismatch between components and cell degradation. In order to permit functionality at lower temperatures, second generation SOFCs which are anode supported were developed later [2] . The thickness of the electrolyte layer in this type of fuel cell can be as thin as 10 μm, with the thick anode providing the mechanical support. The ohmic and activation polarization losses in the electrode supported category are smaller than those contributions in the electrolyte supported cells. The primary problem faced in the operation of anode-supported tubular fuel cells is the effects of redox cycling. During anode oxidation-reduction reactions, Ni oxidizes to NiO, resulting in a volume expansion. This expansion contributes to the formation and propagation of micro-cracks within the Ni-YSZ anode support. Ultimately, this will lead to cell failure [3]. Any accidental oxidation of the anode, mainly caused by a shut down due to system maintenance, on the system, damages the anode-supported fuel cells. In our research the third generation of tubular solid oxide fuel cells which is thought to solve the redox cycling problem is introduced. This type of ceramic fuel cell consists of a slip cast porous support of approximately 500 µm thickness made of calcined YSZ coated with a thin dense YSZ electrolyte [4]. The electrolyte can be coated inside or outside the porous support giving flexibility for the electrode position arrangement. The porous support can be impregnated with nano-sized Ni anode material to solve the redox-cycling problem. The fuel cell configurations before and after impregnation with Ni particles are shown in Figure 1.
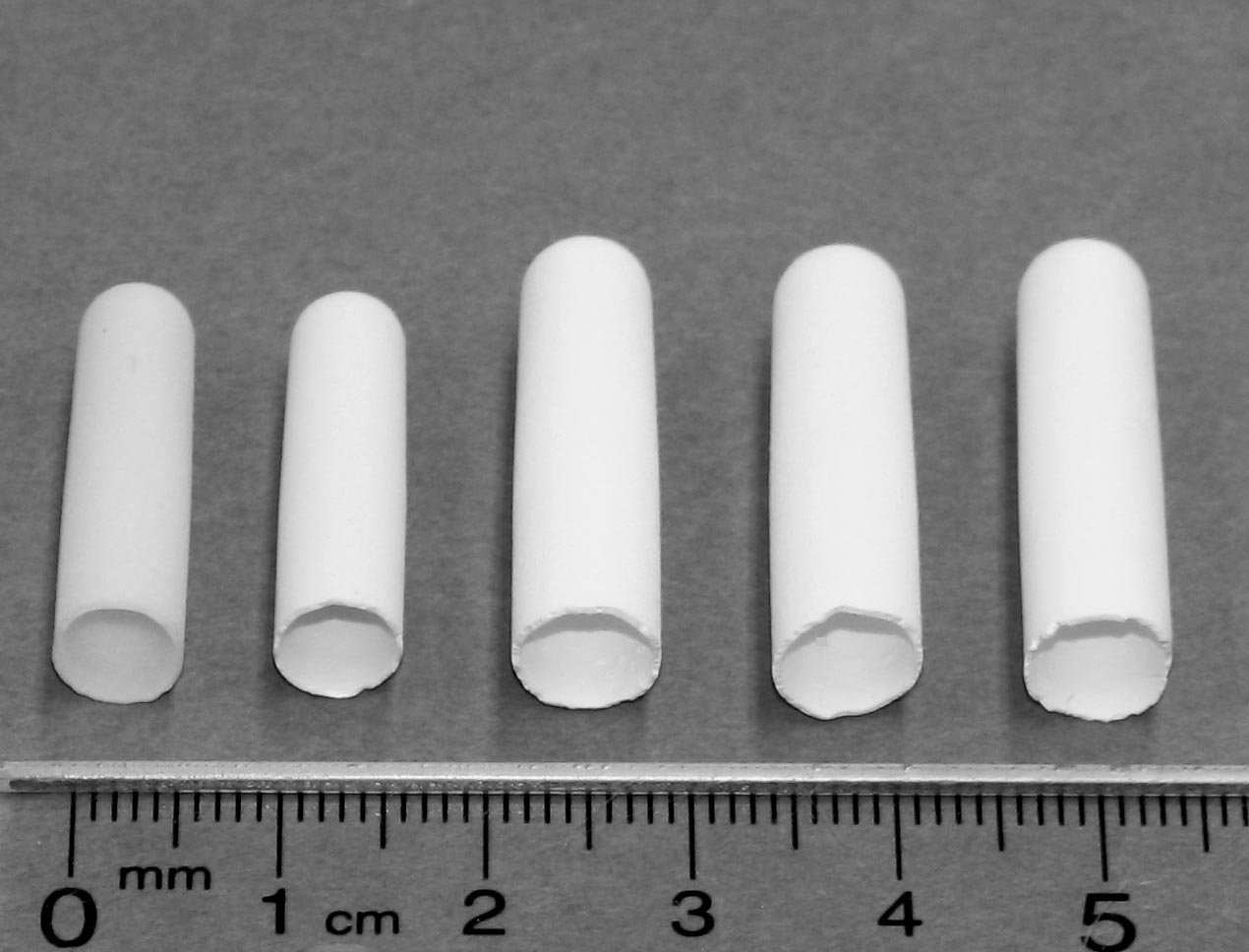
Figure 1. Porous YSZ electrolyte-supported SOFC coated with a dense YSZ and a thin porous cathode layer: (a) before impregnation with Ni, (b) after impregnation. (schematic). The electrochemical performance and redox cycling of the fabricated fuel cells indicate excellent power density and redox cycling resistance. Also these fuel cells show acceptable long term stability and greatt thermal shock resistance. Porous electrolyte supports (PES) are successfully fabricated via slip casting using YSZ calcined at 1300–1500°C with graphite as a pore former and a thin dense layer of YSZ was coated inside/outside the porous support to function as the cell electrolyte. Our research group can produce closed-end tubes with a dimension range 20-150 mm long and 5-10 mm diameter. Figure 2 shows examples of small tubes produced in our lab using a slip casting method.
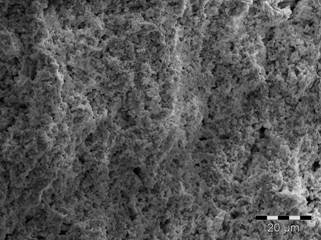
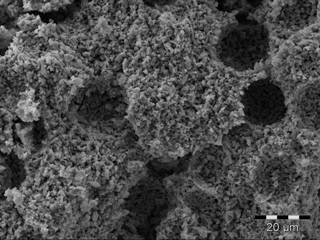
Figure 2. Tubes (from left to right) made of Tosoh YSZ, Tosoh YSZ + 20 vol% graphite, CYSZ 1300, CYSZ 1400 and CYSZ 1500 after sintering at 1350°C. CYSZ: Calcined YSZ. Figure 3 a and b show the microstructure of porous supports made of YSZ calcined at 1500°C, 20 vol% graphite and YSZ calcined at 1500°C, 20 vol% PMMA.
Figure 3. Secondary electron beam SEM images of the YSZ tubes (cross-section), sintered at 1350°C. (a):CYSZ 1500, 20 vol% graphite and (b) CYSZ 1500, 20 vol% PMMA. The electrolyte thickness varies from 10 to 30 µm depending on the solid loading of the electrolyte slip and the coating time (Figure 4). The attachment between the porous support and the thin electrolyte appears excellent in most cases and no cracking or delamination was observed.
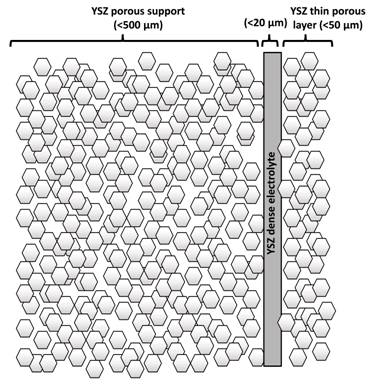
Figure 4. YSZ calcined at 1500°C + 20 vol% graphite coated with electrolyte Collaboration on Infiltration of the New Tubular Design Figure 5 shows the structure of YSZ layers designed for collaboration purposes. The porous support is coated with a thin electrolyte and a thin porous layer. The support and the thin porous layers can be infiltrated with any novel anode/cathode materials. We can provide limited cells based on the following design to the interested research groups whom wish to try their materials in a tubualr based cell. We might be able to give comments about the infiltration procedure of these cells and also performance testing.
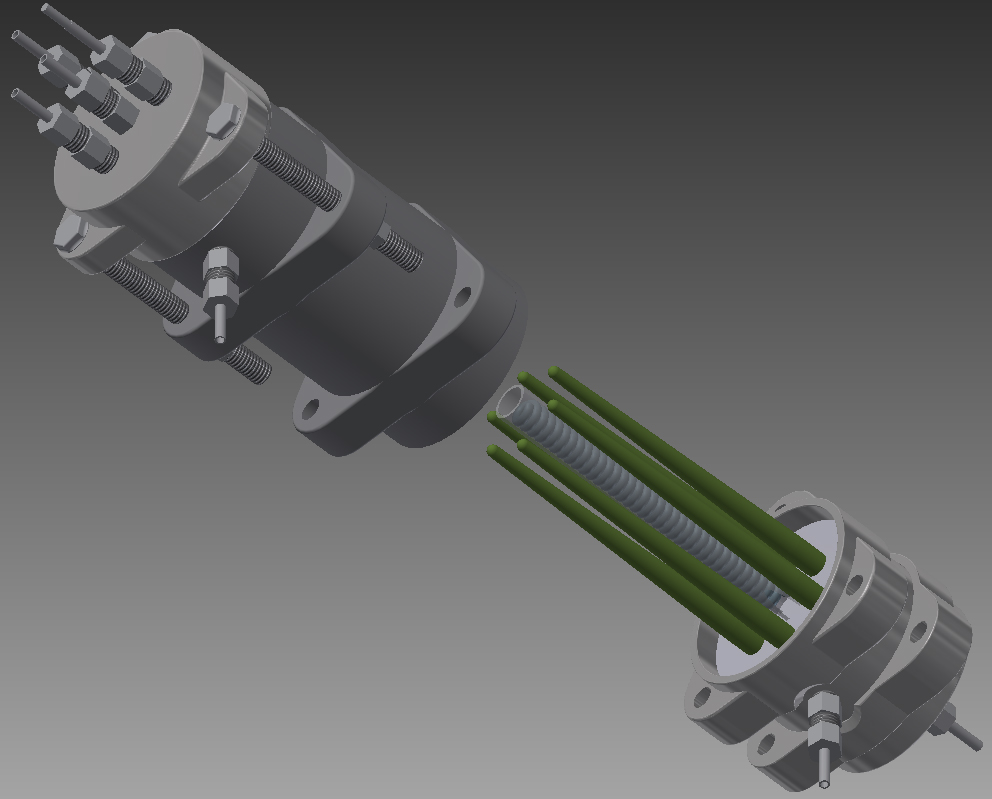
Figure 5. Schematic of the YSZ porous support coated with a dense electrolyte and a thin porous layer. Interested researchers can contact me about a possible collaboration. We have been collaborating on development of tubular fuel cells with Alberta-Innovates Technology Futures, Birss's group at University of Calgary, SOFC groups at University of Alberta, Kendall's group at University of Birmingham, and Tucker's group at Lawrence Berkeley National Laboratory. Currently we are working on development of the first made in Canada tubular SOFC stack using 6 long one end closed fuel cells (Figure 6).
Figure 6. SOFC stack design under development at University of Alberta. Refernces 1. K. Kendall, "Novel ceramic designs for fuel cells," In JFCC International Workshop on Fine Ceramics, Elsevier, Nagoya, 1992, 143-48. 2. K. Kendall, G. Sales, "A rapid heating ceramic fuel cell," In 2 nd International Conference on Ceramics in Energy Applications, ed. F. Riley, London, 1994, 55-63. 3. D. Waldbillig, A. Wood, D. G. Ivey, “Thermal analysis of the cyclic reduction and oxidation behaviour of SOFC anodes,” Solid State Ionics 176 (2005) 847-59. 4. A. R. Hanifi, A. Torabi, T. H. Etsell, L. Yamarte, P. Sarkar, “Porous electrolyte-supported tubular micro-SOFC design,” Solid State Ionics, 192, 368-371, 2011. |