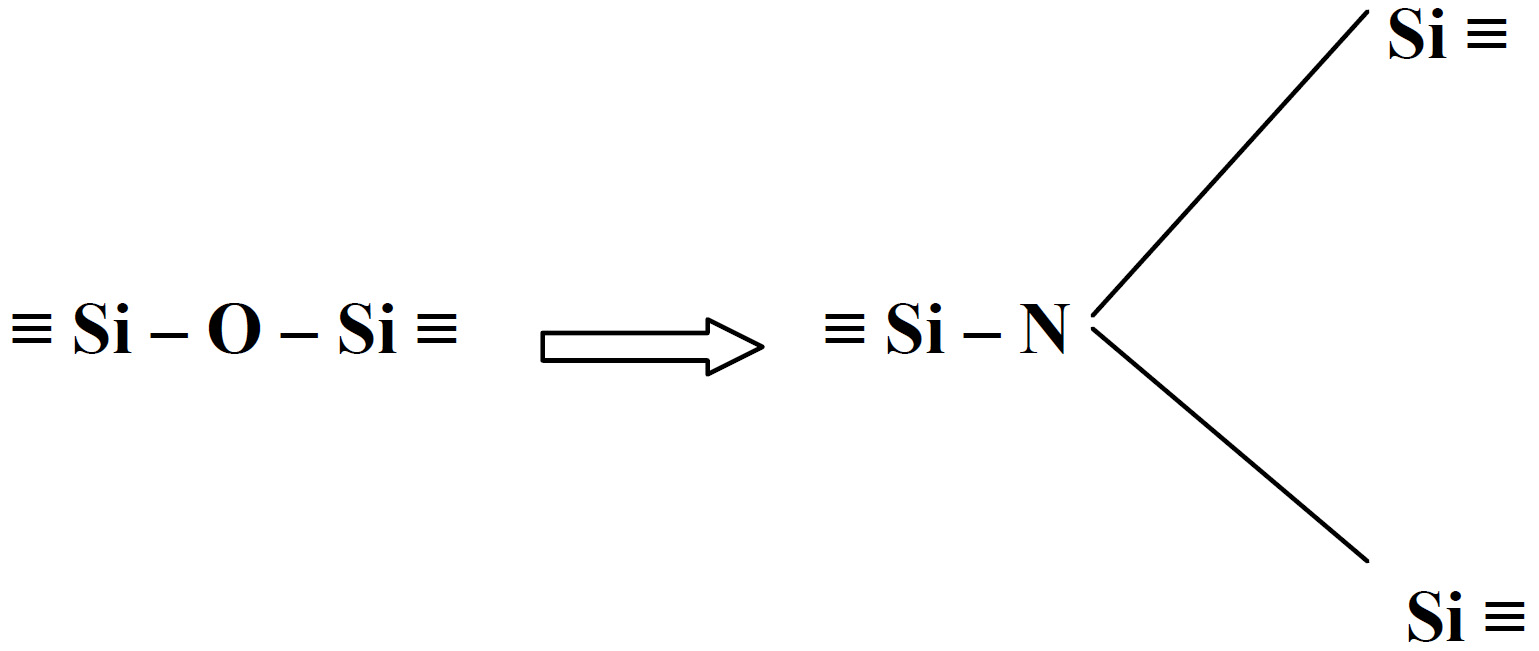Ceramics and Glasses
Ceramics Ceramics are inorganic, non-metallic compounds including most minerals and rocks which cover almost the whole of our planet. They can be divided into two categories of traditional and advanced ceramics. Traditional ceramics had been developed since early human civilisation and include pottery, structural clay products, clay based refractories and cements. Although they represent a major part of the ceramic industry, however, in recent years interests in research and development have mainly focused on advanced ceramics. These ceramics include functional ceramics, used in electronic, magnetic, optical or biomedical applications and structural ceramics, used at ambient or elevated temperatures. Chemically, ceramics are categorised as simple oxides such as alumina (Al2O3) and zirconia (ZrO2), silicates such as kaolinite Al2Si2O5(OH)4 and mullite (Al6Si2O13), non-silicate complex oxides such as barium titanate (BaTiO3). In addition, there are non-oxide ceramics including carbides such as silicon carbide (SiC) and boron carbide (B4C) , nitrides such as silicon nitride (Si3N4) and aluminium nitride (AlN), borides such as titanium diboride (TiB2), silicides such as molybdenum disilicide (MoSi2) and halides such as calcium fluoride (CaF2). There are also compounds based on oxynitride systems (ceramic alloys) such as ß-sialon (Si6-zAlzN8-zOz, where z =4) related to silicon nitride. Glass The word "Glass" comes from an Indo-European root which means "shiny" and also has given us the words glare, glow and glaze. The Latin equivalent phrase for glass is vitreous. The earliest known glaze dates back to 12000 B. C. and the very first glasses were found about 7000 B.C. which were used for decorative objects but later were shaped into vessels. The invention of glass blowing in the first century B. C. increased the applications of glasses for practical purposes such as vessels and windows in Roman times. After the fall of Roman Empire, glass manufacturing was dispersed to isolated sites but was continued in Byzantium and later in the middle-east. Glasses are traditionally made of inorganic materials such as silica sand, sodium and calcium carbonates, feldspars, borates and phosphates that melt together and cool to form solid transparent materials (Morey, 1954; Doremus, 1994). According to the definition of ASTM standards for Glass, "glass is an inorganic product of fusion which has been cooled to a rigid condition without crystallisation". However, it is identified that glass compositions are not limited only to inorganic materials and organic polymers can form glass. Jones (1956) mentioned that "A glass is a material formed by cooling from the normal liquid state, which has shown no discontinuous change at any temperature range, but has become more or less rigid through a progressive increase in its viscosity". Melting is not the only route to obtain a glass and other methods such as vapour deposition, sol-gel processing of solutions and neutron irridation of crystals can lead to glass formation. Whatever the chemical compositions, glasses have two common characteristics: firstly they do not have long range order, that is, there is no regularity in the arrangements of its molecular constituents on a scale larger than a few times the size of these units. For example the average distance between two silicon atoms in vitreous silica (SiO2) is 3.6Å and no order above 10Å is found between these atoms. Secondly they exhibit a time-dependant behaviour known as glass transition phenomenon (Fig. 1) which occurs over a temperature range called the glass transition temperature. Therefore a glass can be defined as an amorphous solid completely lacking in long range, periodic atomic structure and exhibiting a region of glass transformation behaviour. Any material, organic, inorganic or metallic formed by one of the stated processes which shows glass transition behaviour can be categorised as glass (Shelby, 1997) .
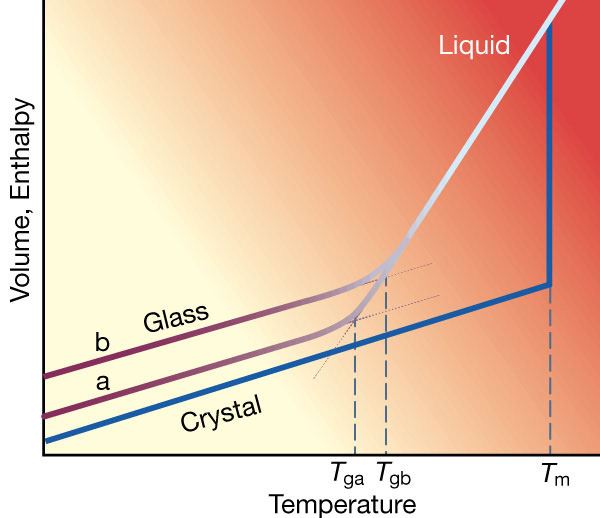
Fig. 1: Effect of temperature on volume or enthalpy of a crystal and a glass cooled. Oxynitride Glasses
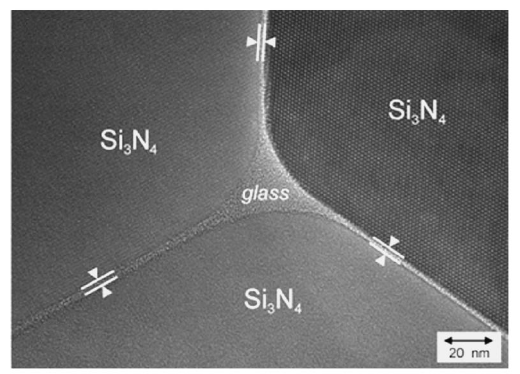
High resolution TEM micrograph of silicon nitride showing a triple-point and Jack (1976) noticed the similarity between the structure of SiO4 units in silicates and SiN4 units in silicon nitride in which oxygen or nitrogen atoms are tetrahedrally oriented around the central silicon. Furthermore, he proposed that due to the similarity between the bond lengths of Si-N (0.174 nm), Si-O (0.170 nm) and Al-O (0.175 nm), nitrogen can enter the structure of alumino-silicate units. Replacement of Si (4+) by Al (3+) and N (3-) by O (2-) occurs according to: which leads to the formation of new nitrogen ceramics and glasses. Vitreous and crystalline materials based on (Si,Al)(O,N)4 tetrahedral building units are called sialons. Sialons are alumino-silicates in which nitrogen is incorporated into their structures and, in the case of charge compensation, other cations such as (Mg (2+), Ca (2+), Sr (2+), Y (3+) etc) can enter the structure to keep the electro-neutrality. The tetrahedral (Si,Al)(O,N)4 units can be joined together through sharing their corners (O,N) and forming networks, sheets, chains or isolated units which are structurally similar to silicate units. The incorporation of modifier cations to the sialons builds structures of greater diversity. These types of materials have the potential to be used as ceramics for wear and high temperature resistance applications. However, if they are melted and cooled at an appropriate rate, due to the high viscosity and low tendency for crystallisation they can form oxynitride glasses (Drew, 1986). Extensive research has been carried out to study the glass formation in different M-Si-O-N and M-Si-Al-O-N systems where M is a modifier element such as a divalent cation (Mg, Ca, Ba), trivalent cations (Y) and also lanthanide elements. The systematic substitution of oxygen by nitrogen results in improvements of physical, chemical, mechanical and thermal properties of M-Si-O-N and M-Si-Al-O-N glasses (M=Ca, Mg, Sr, Na, Y, Ln, etc). Significant studies have been carried out to date that clarify that an increase in density, compactness, chemical durability, refractive index and dielectric constant occurs when nitrogen is substituted for oxygen. Furthermore, glass transition temperature, dilatometric softening temperature, viscosity, Young’s and shear moduli and fracture toughness of these glasses rise when oxygen is replaced by nitrogen and decreases in the thermal expansion coefficient and molar volume are reported after this substitution. Therefore as a result of this transformation from oxide into oxynitride glass which occurs with nitrogen substitution for oxygen, glass becomes more viscous, denser, harder and more refractory. The theory proposed by Mulfinger (1966) can explain the reason for these improvements in the glass structure. When divalent oxygen is replaced by trivalent nitrogen, the average coordination number of the nonmetal atoms increase which results in an increase in the number of linkages between anion, in this case N, and network formers. This increase of cross-link density improves the network connectivity and a more rigid glass is obtained as a result. This is shown schematically in Figure 3. Nitrogen can be bonded to Al as well as to Si and, with greater cross linking, a stronger network is produced.
Fig. 3: Substitution of nitrogen for oxygen and increase in the cross-link density of the Figure 4 is a representation of the M-Si-Al-O-N oxynitride glass structure suggested by Bunker et al. (1987). In this structure Si (4+) and Al (3+) are the network former cations which appear in tetrahedral positions and are interlinked together through bridging oxygen or nitrogen atoms. Depending on the nitrogen concentration, Si(O4), Si(O3N) and Si(O2N2) structural units can form in the network. The network modifier cation (M(3+)) charge balances the extra negative charge on [AlO4]- tetrahedra in which case it is a “network dwelling” cation, but also acts as a network modifier if the ratio of M:Al>1:3, in which case it creates non-bridging oxygen species and lower coordinated N atoms.
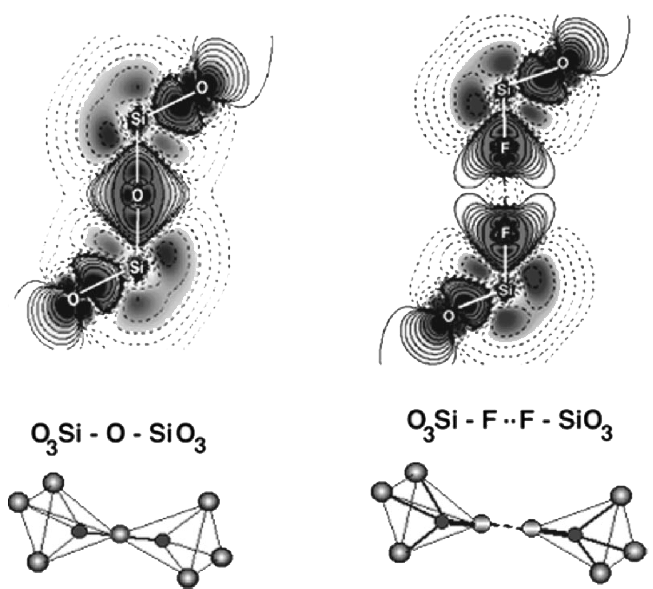
Figure 3. Schematic representation of oxynitride glass structure (Bunker et. al 1987). Oxyfluoride Glasses Oxyfluoride glasses are glasses of different cation compositions (Si, B, Ge, P, alkaline, alkaline earth, rare-earth, heavy metals etc.) containing oxygen and fluorine. Fluorine is used in these types of glasses for a variety of purposes e.g. for production of machinable glasses and glass-ceramics and for opacifying of opal glasses. Due to the lowering of the refractive index, fluorine is widely used in silica waveguides. Heavy metal fluoride glasses (HMFG) have been of significant interest for their interesting optical properties. Oxyfluoride glasses also have the potential for use as UV transmission and laser hosts. Transparent oxyfluoride glass-ceramics doped with rare-earth elements are suitable candidates for optoelectronic devices. Oxyfluoride glasses are used as glass polyalkenoate cements in dentistry where fluorine facilitates glass degradation due to disruption of the network and also enhances the formation of fluorine complexes in a poly salt matr ix. Fluorine release is known to be crucial in attacking dental caries and curing them and this element is also the most effective agent in caries prevention. This occurs through inhibition of the mechanisms leading to the formation of caries as well as reinforcing the enamel and dentins against bacterias. Formation of fluoroapatite which is desirable for biomedical applications is observed after crystallisation of high fluorine content SiO2-Al2O3-P2O5-CaO-CaF2 glasses. This crystallisation occurs homogeneously through phase separation of the glass (Stanton and Hill, 2000; Stanton and Hill, 2005). Fluorine, which is the most electronegative element of the periodic table, is known to be a powerful network disrupter, which is substituted for bridging oxygens (because the ionic radii of these two elements are similar), in the structure of glasses, forming non-bridging oxygen (NBO) and fluorine atoms. As a result of this destructive substitution, glass viscosity and the glass transition temperature (Tg) decrease drastically. Fluorine also aids crystallisation by lowering the viscosity which causes a better mobility of atoms and rearrangement of them to form crystals. Fluorine usually acts as a nucleating agent and as it promotes melt crystallisation, oxyfluoride glasses are less stable compared with other glasses. The thermal expansion coefficient and electrical conductivity of oxyfluoride glasses rise with increasing the amount of fluorine (Shelby and Lord, 1990). Fluorine also has an important influence on the phase separation of glasses and subsequent bulk crystallisation. It also reduces refractive index of the glasses and contributes to the elimination of OH- groups (Rafferty et al. 2000). Figure 5 shows schematically the effect of replacement of oxygen by fluorine in silicon centred tetrahedra on the overall charge density difference. Solid lines represent increased charge density and dashed lines denote decreased charge density. The difference between the electronegtivities of Si and F is greater than that between Si and O, and thus Si-F bonds are more ionic and stronger than Si-O bonds (calculations show that the fluorinated unit has 1.5 eV higher energy than SiO4 unit). Therefore, with fluorine substitution for oxygen, the internal attractive force between anions and Si increases. However, there is a powerful Coulomb repulsive force between SiO3F groups which leads to the separation of these units while oxygen bridged SiO4 groups, are linked strongly. This phenomenon leads to a decrease of cross linking in the system and reduction of melt viscosity (Painter et al., 2002).
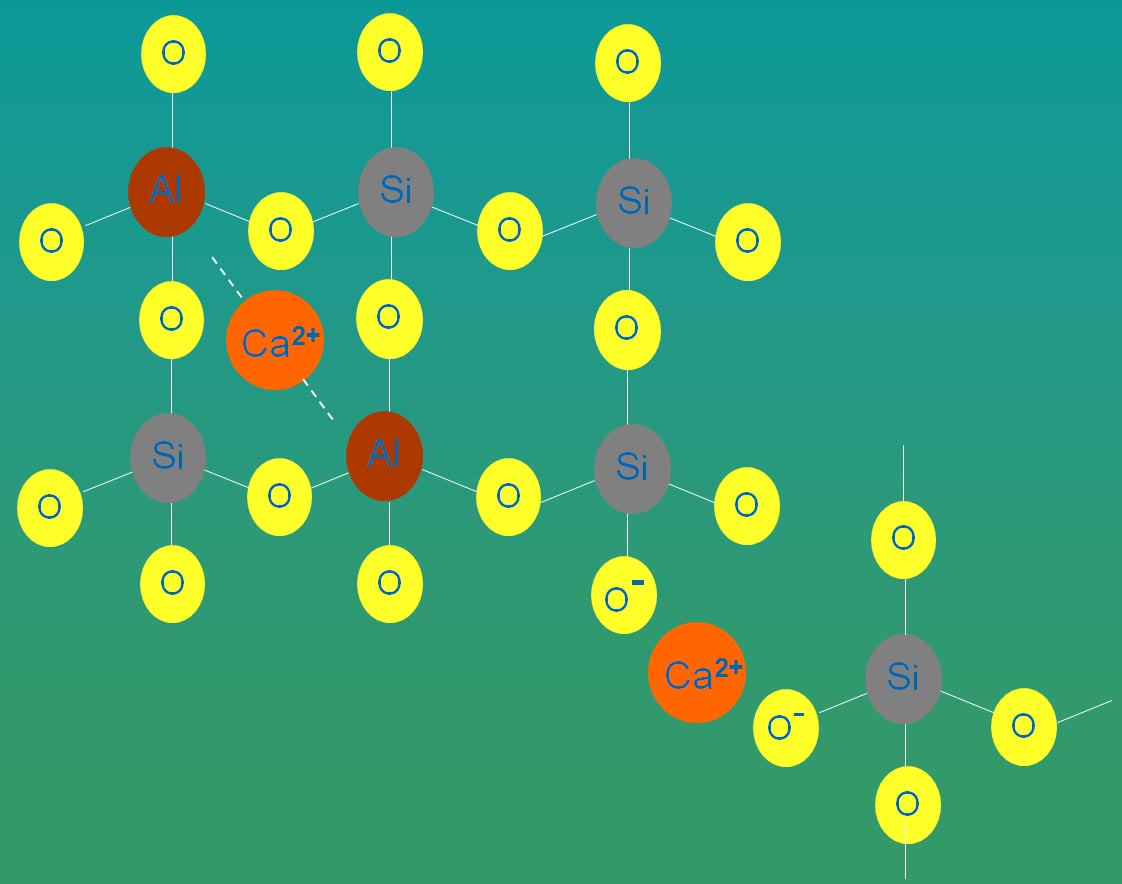
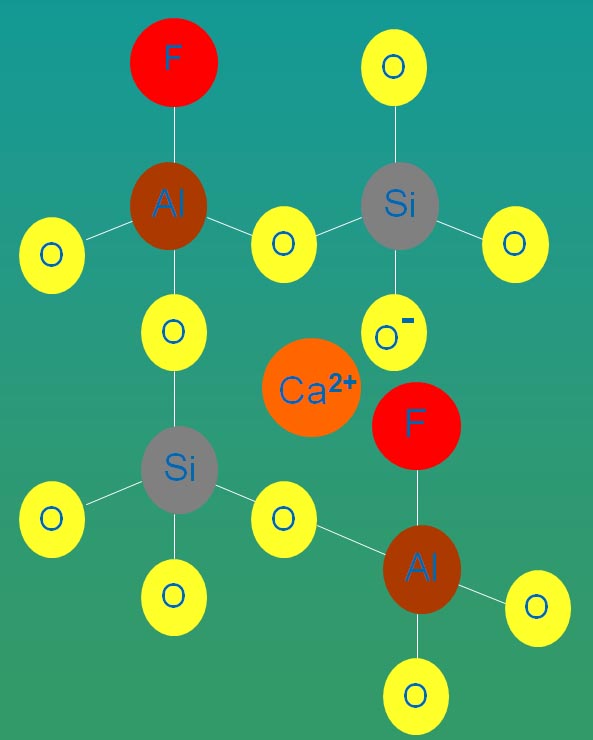
Fig. 5: Charge density difference between SiO4 tetrahedra (left) and the same cluster Fluorine content has been shown to have a strong effect on the reduction of the glass transition temperature in a variety of glass systems such as fluoro-alumino-silicate, calcium-fluoro-aluminate,phosphor-fluoro-alumino-silicate,phosphorfluoro-calcium alumino-silicate , phosphor-fluoro-silicate and fluoro-silicate glasses. It is thought that there is a direct relationship between the term “cross-link density” of the network and the glass transition temperature. Whereas fluorine replacement for oxygen disrupts the network and decreases the cross-link density, the energy required to move the glass structural units reduces and as a result, the glass transition temperature decreases (Hill et al., 1999).The schematic structure of a calcium-alumino-silicate glass (Fig. 6) mixed with CaF2 to form an oxyfluoride glass (Fig. 7) are shown. It may be seen that the Si and Al centred tetrahedra of the oxide glass are linked together through bridging oxygens and Al tetrahedra are charge balanced by the modifier Ca (2+) ions. Incorporated fluorine substitutes the BO and is bonded preferentially to Al which can keep it in the structure and prevent its loss. The NBOs are mainly associated with Si that together with [AlO4]- and [AlO3F]- tetrahedra are charge compensated by the modifier cation, Ca (2+).
Fig. 6: Schematic structure of calcium-alumino-silicate glass.
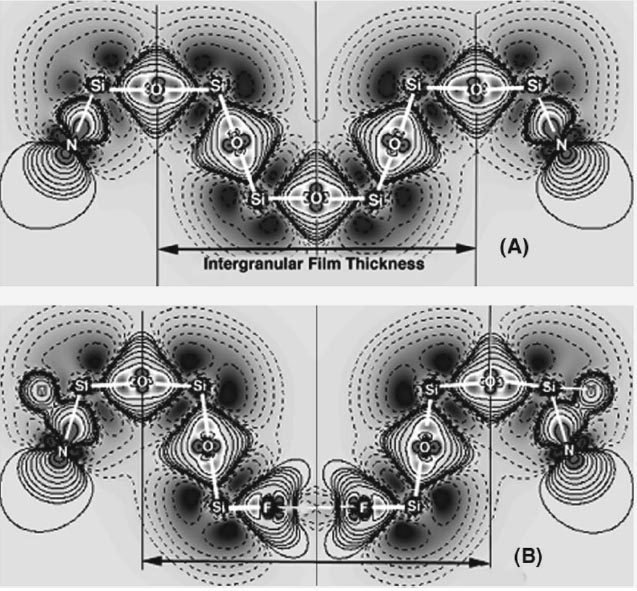
Fig. 7: Schematic structure of calcium-alumino-silicate glass. Oxyfluoronitride Glasses Oxynitride glasses containing fluorine which form in the triple points and the grain boundaries of nitrogen ceramics such as Si3N4 are called oxyfluoronitride glasses. The thickness of the intergranular film (IGF) is controlled by the compression due to the attractive forces between the grains and the repulsion of the atomic structural units. A silica-rich IGF usually has a thickness of 1 nm where with incorporation of dopants like fluorine it expands to 1.1 nm. The expansion of the IGFs occurs due to two reasons which are the breakage of the network by incorporation of the monovalent anion as well as the Coulomb repulsion force between the anions (Kleebe and Pezzotti, 1998). Figure 8 represents a calculated charge density difference in major symmetry plane of the IGF where in the first image, a Si6O13N6 cluster links two Si3N4 grains. The bridging oxygen has linked the two Si tetrahedra together but with replacement of fluorine for oxygen as shown in the second picture the Si tetrahedral are separated and there is a repulsive force between the two fluorine atoms. This substitution leads to a reduction of viscosity and an increase of 0.1 nm in thickness of the IGF.
Fig. 8: Calculated charge density difference in a symmetry plane of IGF of Si3N4 (A): In the history of oxynitride glasses, there is not much known about oxyfluoronitride systems and their properties. Vaughn and Risbud (1984) incorporated nitrogen into Zr-Ba-Al-Y-O-F glasses in order to increase the thermal stability and also mechanical propertiesof these oxyfluoride glasses and obtained some increase in glass transition and crystallisation temperatures as well as hardness as a result. Wusirika and Chyung (1980) also added AlF3 to some different oxynitride glasses in order to lower the melting temperature and enhancing the crystallisation of nitrogen containing phases. Fletcher and Risbud (1988) used nitrogen to increase the glass stability as well as chemical durability of some fluorophosphate glasses in the M-Al-P-O-F-N system (M=Ba,Na). In fact oxyfluoronitride glasses can be viewed from two aspects. First, they can be assumed as oxyfluoride glasses in which nitrogen is incorporated with the aim of improving their mechanical properties, thermal stability and chemical durability. Therefore the interesting properties of oxyfluoride glasses such as machinability, UV transmission, biomedical properties, etc., can be reinforced mechanically, thermally and chemically by nitrogen inclusion. The second approach is to consider them as oxynitride glasses having enhanced mechanical properties and introducing fluorine which reduces their melting point and facilitates the melting condition, which is usually carried out at high temperatures (1600-1800°C). Despite all of these efforts to improve the properties of oxyfluoride glasses using nitrogen or lowering the sintering temperature of oxynitride glasses using fluorine, there has been very little systematic investigation of oxyfluoronitride systems including glass formation, properties and crystallisation, mainly when fluorine is added to the M-Si-Al-ON systems. In my PhD research work it was attempted to investigate this system more deeply from different aspects of glass formation, properties and crystallisation. In this regard the other related systems such as oxide, oxyfluoride and oxynitride glasses were studied beside oxyfluoronitride system to assist understanding of this complex system in an easier way. The effects of adding fluorine on glass formation and stability, melting temperature, phase separation and nitrogen solubility and the effect of nitrogen on fluorine saturation limit in oxyfluoronitride systems were studied. Also information about physical, mechanical and thermal properties of glasses were provided by changing the anions ratio at constant cations ratio and vise versa. The effects of varying anions and cations systematically on crystallisation behaviour including crystalline phases and microstructure of glass-ceramics and their physical and mechanical properties were studied and finally the potential application of the developed glasses and glass-ceramics for biomedical purposes was evaluated. Further information about this secion is available upon your request. Refernces Bunker, B. C., Tallant, D. R., Balfe, C. A., Kirkpatrick, R. J., Turner, G. L. & Reidmeyer, M. R. (1987). Structure of phosphorus oxynitride glasses. J. American Ceramic Society, 70(9), 675–681. Doremus, R. H. (1994). Glass Science, John Wiley & Sons Inc., New York. Drew, R. A. L. (1986). Nitrogen Glass, The Parthenon Press, UK. Fletcher, J. P. & Risbud, S. H. (1988). Preparation of Oxyfluoronitride Glasses from Fluorophoshate-AlN Metals. Materials Letters, 6(5.6), 145-148. Hill, R., Wood, D. & Thomas, M. (1999). Trimethylsilylation analysis of the silicate structure of fluoro-alumino-silicate glasses and the structure role of fluorine. J. Materials Science, 34, 1767-1774. Jack, K. H. (1976). Review-Sialons and Related Nitrogen Ceramics. J. Materials Science, 11(6), 1135-1158. Jones, G. O. (1956). Glass, Methuen, London. Morey, G. W. (1954). The Properties of Glass, Reinhold, New York. Mulfinger, H. O. (1966). Physical and chemical solubility of nitrogen in glass melts. J. American Ceramic Society, 49(9), 462-467. Painter, G. S., Becher, P. F., Kleebe, H. J. & Pezzotti, G. (2002). First-principles study of the effects of halogen dopants on the properties of intergranular films in silicon nitride ceramics. Physical Review, 65(6), 1-11. Rafferty, A., Clifford, A., Hill, R., Wood, D., Samuneva, B. & Dimitrova-Lukacs, M. (2000). Influence of Fluorine Content in Apatite Mullite Glass Ceramics. J. American Ceramic Society, 83(11), 2833-2838. Shelby, J. E. (1997). Introduction to Glass Science and Technology, Athenaeum Press Ltd, UK. Shelby, J. E. & Lord, C. E. (1990). Formation and Properties of Calcia-Calcium Fluoride-Alumina Glasses. J. American Ceramic Society, 73(3), 750-752. Stanton, K. & Hill, R. (2000). The role of fluorine in the devitrification of SiO2.Al2O3. P2O5.CaO.CaF2 glasses. J. Materials Science, 35, 1911-1916. Stanton, K. T. & Hill, R. G. (2005). Crystallisation in apatite-mullite glass–ceramics as a function of fluorine content. J. Non-Crystalline Solids, 275, e2061-e2068. Vaughn, W. L. & Risbud, S. H. (1984). New fluoronitride glasses in zirconium-metal-F-N systems. Materials Science Letters, 3, 162-164. Wusirika, R. R. & Chyung, C. K. (1980). Oxynitrid Glasses and Glass-Ceramics. J. Non- Crystalline Solids, 38 & 39, 39-44. |