Tutorial
The following is a brief introduction to inhaled pharmaceutical aerosols (IPAs). For a more complete description, see for example "The Mechanics of Inhaled Pharmaceutical Aerosols: An Introduction", by W. H. Finlay, Academic Press, 2nd edition, 2019, or "Pharmaceutical Inhalation Aerosol Technology" edited by A. J. Hickey, Marcel Dekker, 3rd edition, 2019.
Inhaler Types
There are many different inhalers used to deliver IPAs. Most people have seen someone using a "puffer" to alleviate asthma symptoms. The technical term for such puffers is "pressurized metered dose inhaler" or "propellant metered dose inhaler", usually abbreviated as pMDI or simply MDI. A sketch of a typical such device is shown below in Figure 1.
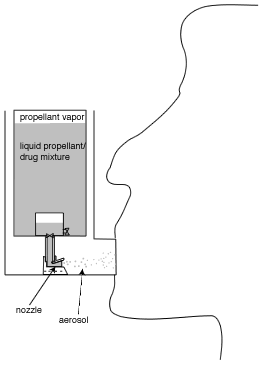
pMDIs are the most commonly used inhaler worldwide, and have been used since the mid 1950s. The aerosol is created when a valve is opened (usually by pressing down on the propellant canister), allowing liquid propellant to spray out of a canister (in a manner somewhat similar to everyday aerosol spray cans), involving a complex process called flashing. The drug is usually contained in small particles (usually a few millionths of a meter in diameter) suspended in the liquid propellant, but in some formulations the drug is dissolved in the propellant. In either case, the propellant evaporates rapidly as the aerosol leaves the device, resulting in small drug particles that are inhaled. Prior to the mid 1990s, pMDIs used various chlorofluorocarbons (CFCs) as their propellant, but with the elimination of CFCs in industry due to ozone depletion concerns, the propellants in current pMDIs use hydrofluoroalkanes (HFAs), which do not result in ozone depletion.
Add-on devices (spacers, holding chambers and other modifications) are sometimes used with pMDIs (particularly in young children) to remove the difficulty some patients have with coordinating inhalation with firing the pMDI spray. Such add-on devices can also reduce the amount of drug depositing in the mouth and throat.
Although pMDIs are the most common type of modern day inhaler, they are not the oldest. This honour goes to nebulizers, which have been around in one form or another for more than a century. Nebulizers produce a mist of drug-containing water droplets for inhalation. They are usually classified into two types: electronic nebulizers and jet nebulizers. Jet nebulizers are more common due to their lower cost, and use a source of pressurized air to blast a stream of air through a drug-containing water reservoir, producing droplets in a complex process involving a viscosity-induced surface instability that leads to nonlinear phenomena in which surface tension and droplet breakup on baffles play a role. In contrast, electronic nebulizers produce droplets by mechanical vibration of a plate or mesh. In either type of nebulizer, the drug is usually contained in solution in the water in the nebulizer and so the droplets being produced contain drug in solution. However, for some formulations (notably Pulmicort) the drug is contained in small particles suspended in the water, which are then contained as particles suspended inside the droplets being produced. A schematic of a typical nebulizer is shown in Figure 2.
The third major type of inhaler is the dry powder inhaler, abbreviated as DPI. In DPIs the aerosol is a powder, contained within the device until it is inhaled. The therapeutic drug is manufactured in powder form as small powder particles (usually a few millionths of a meter, or micrometers, in diameter). In some DPIs the drug is mixed with much larger sugar particles (usually lactose monohydrate), that are typically 50-100 micrometers in diameter. The much smaller drug particles attach to these excipient particles. The increased aerodynamic forces on the lactose/drug agglomerates improve entrainment of the small drug particles upon inhalation, in addition to allowing easier filling of small individual powder doses. Upon inhalation, the powder is broken up into its constituent particles with the aid of turbulence and/or mechanical devices such as screens or spinning surfaces on which particle agglomerates impact, releasing the small, individual drug powder particles into the air to be inhaled into the lung. The sugar particles are intended to be left behind in the device and in the mouth-throat.
Although pMDIs, DPIs and nebulizers are the three major types of inhalers currently on the market today, new inhaler designs are continually being invented and several novel designs are now available or making their way through the regulatory approval process, so that some inhalers that do not readily classify into one of the three traditional inhaler types mentioned above.
The Importance of Particle Size
When the lung is the target for the aerosol (either because the intent is to treat the lung surface or to get the drug into the blood through the capillaries via the alveoli) the inhaled aerosol must consist of particles in a certain size range. This is because particles larger than a certain size tend to simply land in the mouth and throat and mostly do not make it into the lung (like a big truck travelling too fast around a corner, they have too much mass to make it around the bends in the convoluted path through the mouth and throat). Particles somewhat smaller than a certain size tend to get inhaled and then exhaled right back out, while very small particles usually can't be made in high enough numbers to give high enough dosages. Thus, IPAs are usually designed to produce drug particles each having the incredibly small mass of between approximately 1 trillionth and 100 trillionths of a gram. For particles with densities near that of water, this corresponds to particle diameters of a few millionths of a meter (i.e. a few micrometers). For example, the figure below shows the probability that inhaled droplets of different diameters will deposit in the mouth-throat ("extrathoracic"), tracheobronchial and alveolar regions of the lung for a particular aerosol used in treating asthma (the curves in the figure below will be different for a different aerosol and cannot be used to evaluate other aerosols than the one shown, particularly since the aerosol shown undergoes drug specific evaporation in the lung).
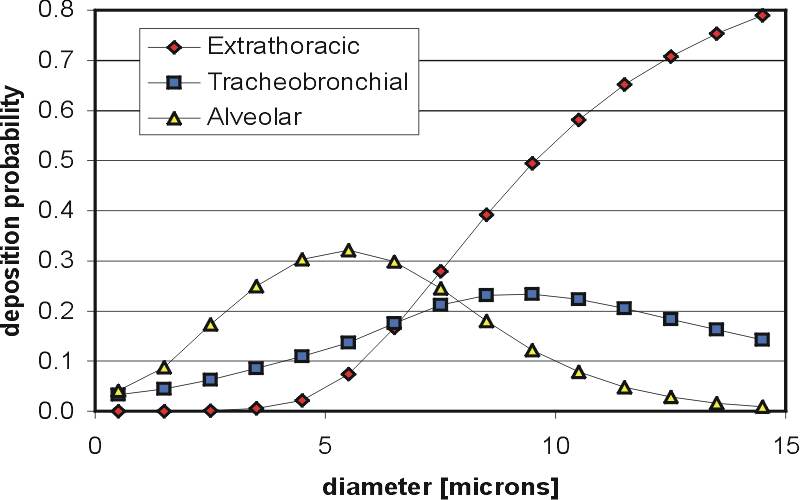
Although specific size ranges are often quoted as being ideal for IPAs (e.g. 1-5 micrometers in diameter), significant amounts of particles outside this size range can deposit in the lung, so that these size ranges should not be viewed as strict criteria. This is partly because the speed of the inhaled air plays a significant role in determining what size of particles will deposit where in the respiratory tract (so that someone breathing very slowly may cause larger particles to make it deeper in the lung than someone inhaling very rapidly). In addition, the filtering curves for particle deposition are slowly varying functions of particle size, and do not give ideal "bandpass" filtering of particle size, as is clearly seen in the figure above. The reader can use our Deposition Calculator to examine how different parameters affect aerosol deposition in the respiratory tract. Finally, droplet evaporation or condensation can be different for different aerosols and result in different deposition patterns with different aerosols.
Some Challenges with Inhaled Pharmaceutical Aerosols (IPAs)
Scientists and engineers must cope with several factors that make designing an IPA device quite difficult. One of the main challenges with IPAs is producing such small particles - it is not easy to efficiently produce drug particles that have a mass between 1 and 100 trillionths of a gram in a device that be carried around easily! Even if a process is developed that can produce such small drug particles for one particular drug, it may work differently, or not work well, for a different drug. Unfortunately, no general theory is available to predict the behaviour of different drugs in different devices, so the research and development process must be revisited for each drug under consideration, a time-consuming and labor intensive process.
Another challenge is caused by the fact that everyone inhales differently. Thus, the speed of the air that an inhaled pharmaceutical aerosol (IPA) is exposed to is different for everyone. This presents some interesting challenges. For example, for certain dry powder inhalers, this requires trying to design the powder so that people inhaling either slowly or quickly both inhale similar amounts of powder from the device. Inhaled air speed can also affect where particles deposit in the respiratory tract, as can clearly be seen by using our Deposition Calculator to examine the effect of different flow rates on aerosol deposition in the respiratory tract.
A second complicating factor is caused by differences in the geometry of people's mouth-throat airway passages, with some people having very sudden bends that cause many particles to deposit in the mouth-throat while others have a relatively "smooth" mouth-throat passage with much less mouth-throat deposition. This can cause variability in the dose reaching the lung between different patients. A further complication is added by the differing effects of device mouthpieces on mouth-throat geometry and their effect on the fluid mechanics in the mouth-throat (small diameter mouthpieces that have been commonly used in the past have been shown by us to cause high mouth-throat deposition due to a high speed jet of air impacting in the mouth). There are also differences in the lung geometry between individuals. Finally, different disease states can also result in differences in where IPAs deposit in the lungs of different individuals.
For drug carried by inhaled water droplets (such as are produced by nebulizers), the humidity of the air being inhaled can cause differing amounts of droplet evaporation and also give rise to variations in where the drug deposits in the lung. In fact, evaporation of nebulized aerosols leads to one of the most commonly made mistakes when measuring nebulized aerosols with a measurement device called a cascade impactor. Users unfamiliar with nebulized aerosols often mistakenly mix the nebulized aerosol with room air during sizing, or else allow the nebulized aerosol to be warmed to room temperature inside the cascade impactor, both of which can lead to significant undersizing of the aerosol (and incorrect assumptions regarding where in the lung the aerosol will deposit).
These are just a few of the challenges that need to be considered with IPAs. Much research goes into overcoming these, as well other challenges not mentioned here. Indeed, the field of IPAs is relatively unexplored in comparison with traditional engineering fields. The Aerosol Research Laboratory of Alberta is one of several laboratories that is helping to explore this interesting and important area.