Past Research
Much of the research performed to date by the Aerosol Research Laboratory of Alberta (ARLA) is presented in publications available in the archival literature. A brief summary of some of this research is given below.
Part of the work done at ARLA has focused on developing ways to control and predict how much drug deposits in the different parts of the respiratory tract with inhaled pharmaceutical aerosols, much of which is incorporated in our Deposition Calculator that allows on-line calculation of respiratory deposition for a variety of relevant parameter values.
Work by ARLA using many replicas of the mouth, throat and nose airways in subjects across the age spectrum has provided a detailed fundamental understanding of mouth-throat deposition with inhalers, allowing improvements in our ability to precisely deliver drugs to the lungs in infants, children and adults. Such methods are useful in helping design and evaluate aerosol delivery devices. Indeed, the "Alberta Idealized Throat" geometry developed by us is used worldwide to evaluate mouth-throat deposition of aerosols delivered by inhalers. Its companion idealized child mouth-throat geometry and idealized infant nasal geometry allow benchtop measurements of aerosol delivery over the complete range of ages from infant to adult. Recently, our work has extended into assessment of nasal drug products. The “Alberta Idealized Nasal Inlet” has been developed to evaluate regional nasal deposition of drugs delivered in nasal sprays.
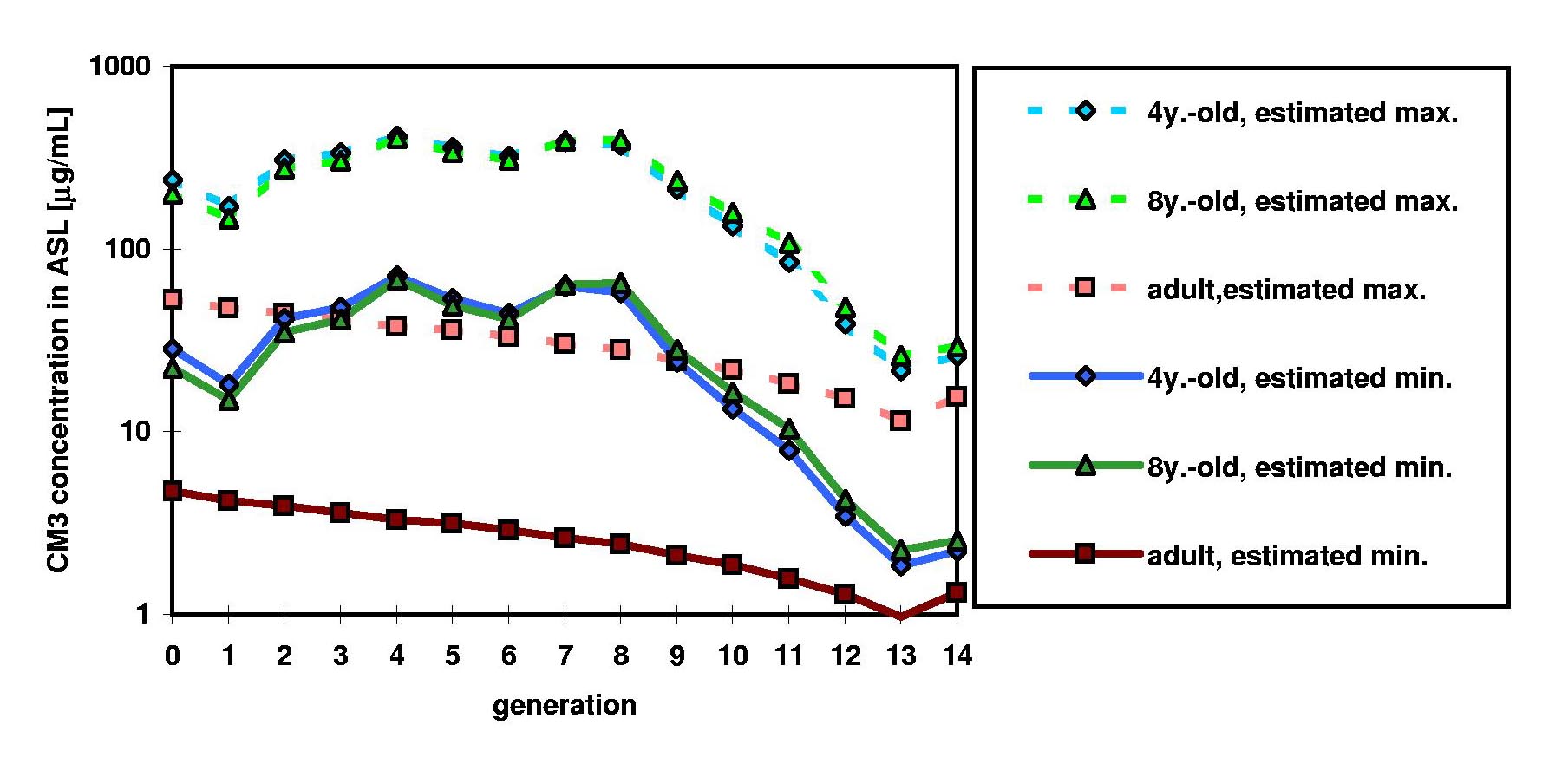
Models developed at ARLA also allow prediction of drug concentrations in the airway surface fluid, now routinely used at ARLA to aid in developing formulations and delivery systems for drugs intended for local activity in the airways (such as antimicrobials and mucolytics, an example of which is shown in Figure 1). These models have been coupled with pharmacokinetic models to predict drug concentrations in the lungs and in systemic circulation over time following administration of inhaled doses.
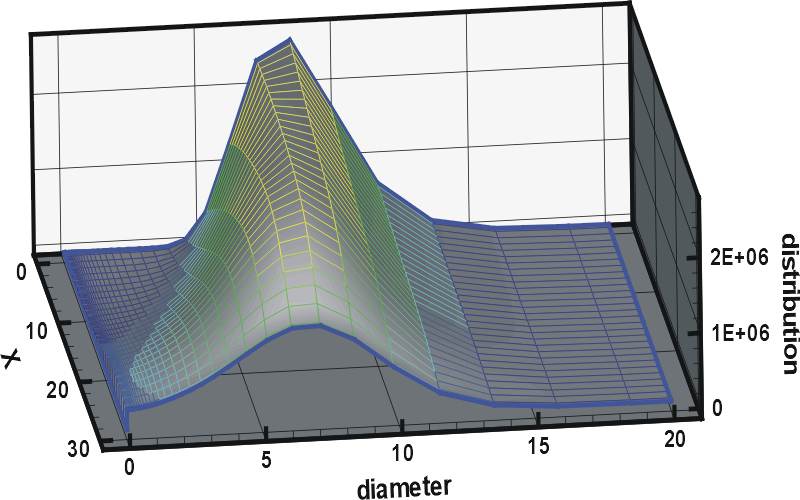
ARLA has also been instrumental in the development of models that allow prediction of evaporation and condensation of inhaled aerosols in the moist environment of the lung, in which the presence of the particles affects the humidity in the lung and vice versa (so-called two-way coupled hygroscopic models, an example of which is shown in Figure 2).
An important part of this work involves validating these models by performing studies to compare to experimental measurements of aerosol deposition in human subjects. A three-dimensional radionucleotide image of the lungs of a patient from one such study at ARLA is shown in Figure 3.

Another part of the research done at ARLA has focused on developing and evaluating delivery systems for various new and existing drugs (an example of one such formulation is shown in Figure 4 below). Much of this work is proprietary, since it involves new technology and formulations that remain undisclosed. However, ARLA has been instrumental in developing various novel experimental methods that allow more realistic characterization of pharmaceutical aerosols during simulated human breathing, allowing exploration and understanding of factors not previously understood, such as the negative effect of humidity on spacers used with ventilated patients, the effect of nebulizer cooling on droplet undersizing in cascade impactors, and the influence of realistic, time-varying inhalation flow rates on aerosol deposition in the mouth and throat.

These novel methods have been used at ARLA for inertial sizing during realistic breath simulation to explore the effect of breath simulation on measurements of aerosols from dry powder inhalers, add-on devices with pressurized metered dose inhalers, as well as nebulizers. In addition, by combining such measurements with our models of aerosol behaviour in the respiratory tract, we are able to optimize and evaluate drug formulations and delivery systems prior to beginning human or animal studies. Indeed, we have collaborated with many companies and agencies, using our methods to help optimize aerosol delivery systems prior to in vivo studies. This work includes extension of these methods to consider aerosols consisting of suspensions (rather than solutions), including liposomes and their associated tendency for disruption. In addition, we have used this approach to allow comparison of many different delivery devices already on the market.
Despite the continuing efforts of ARLA and many other laboratories around the world, much about inhaled pharmaceutical aerosols remains poorly understood, partly due to the complexity of their behaviour. ARLA is part of a strong worldwide community of researchers dedicated to improving our understanding and ability to predict the behaviour of inhaled pharmaceutical aerosols.