Research Associate, 2014-present: University of Alberta, National Institute for Nanotechnology, National Research Council, Edmonton, Alberta, Canada
Post-doctoral Fellow, 2009-2014: University of Alberta, National Institute for Nanotechnology, National Research Council, Edmonton, Alberta, Canada
PhD, Physics, 2009: Kent State University, Kent, Ohio, USA
MSc, Physics: Tribhuvan University, Kirtipur, Nepal
Optical Tweezers
I study biomolecules using optical tweezers. Optical tweezers, highly focused laser beams, apply force to unravel single biomolecules. By measuring the end-to-end distance of a molecule during unfolding, we obtain structural and dynamic information. For this, the molecule of interest (e.g. a protein, RNA, DNA) is attached to DNA handles linked to polytyrene beads that can be manipulated by tweezers. |
Covid-19: programmed ribosomal frameshifting and translation Currently, I am studying corona viruses, focusing on Covid-19. Most viruses, including Covid-19, porduce multiple proteins from one reading frame in its genome by -1 programmed ribosomal frameshifting. In programmed ribosomal frameshifting, ribosome steps back by a nucleotide at a predetermined rate and misses the stop codon (in its genome), producing a new extended protein. The slippage happens at the slippery sequence preceeded by a knot like structure of mRNA called pseudoknot, and the pseudoknot plays important role in this mechanis. The predetermined ratio of such proteins is important for viral propogation. Our goal is to determine the structure of the pseudoknot and the mechanism of frameshifting and then disrput framseshifting by altering the properties of the pseudoknotand. To disrupt the properties of the pseudoknot, we will search a likely drug compound which may disrupt viral propogation.This method will then be extended to other visures, like HIV. |
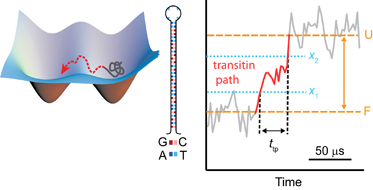
Physical picture of biomolecular folding
I have been studying the folding mechanism of biomolecules. For the first time, I measured directly the transition path of folding in DNA and proteins experimentally. The transition path is a very brief trajectory of a molecue while changing its structure (e.g. while changing to the folded state (F) from unfolded (U)), and contains the critical information about the folding process. |
|
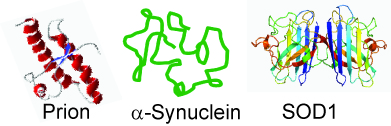
Protein misfolding and aggregation
Protein misfolding and aggregation is associated with many devestating neurodegenerative disorders that have no cure, e.g. Alzheimer's, Parkinson's, and ALS disease. I have been investigating misfolding and aggregation in two proteins: prion (associated with madcow disease) and α-synuclein (associated with Parkinson's disease). Currently, I am trying to directly measure, at the single molecule level, how a misfolded protein converts a correctly folded SOD1 protein (an antioxidant, but associated with ALS disease due to misfolding ) into misfolded form. Despite nobel prize award to the discovery that misfolded prion proteins are infectious (in 1997 to S Pruisner), this infectious mechanism has been diffcult to directly measure at the single molecule level. |
||