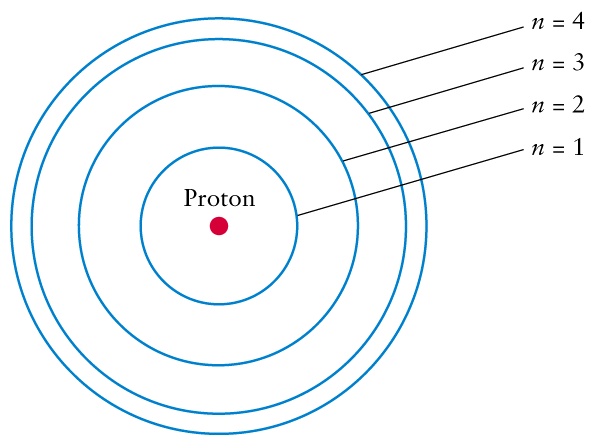
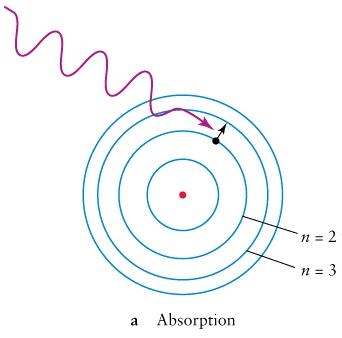
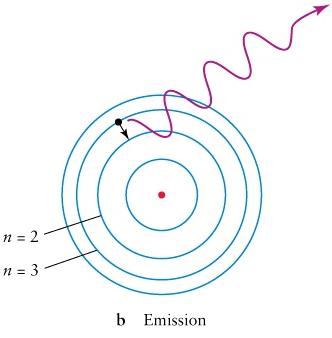
Structure of an Atom
- A typical atom has a diameter close to 10-10 m.
- Most of the mass of an atom is concentrated in a tiny nucleus which has a diameter close to 10-14 m.
- The nucleus is composed of two types of particles: protons and neutrons.
- Electrons move in the region outside of the nucleus.
- We don't know exactly where an electron is, but we can describe the probability that the electron is in a certain region called an orbital.


 ,
,