- Continuous spectrum

- Discrete emission spectra
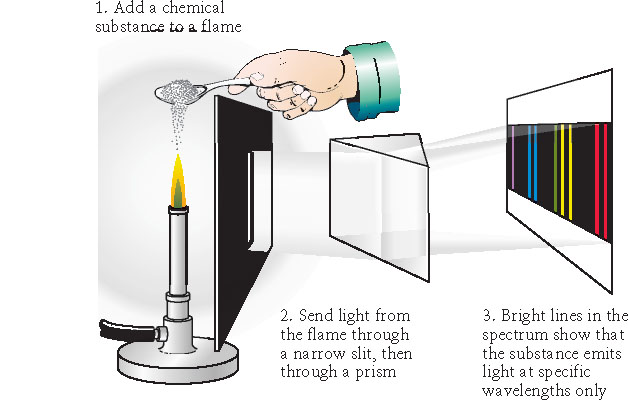
- Discrete absorption spectra
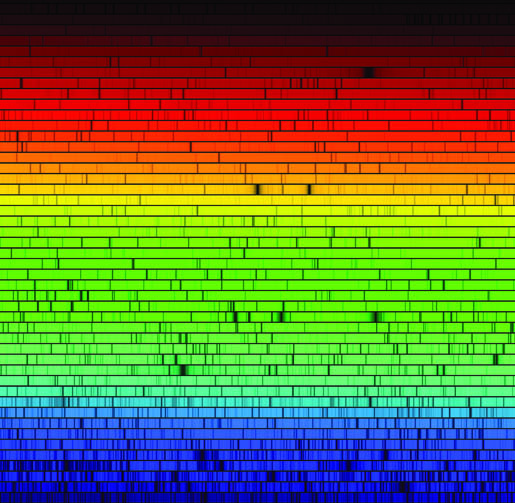

|

|

|
| E.g. blackbody spectrum from a heated source | Emission by heated transparent gas | Stars |
Kirchhoff's Laws
|
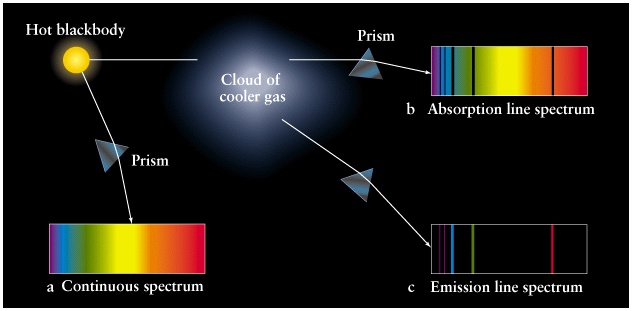 |
Astronomy: A Hydrogen Emission Nebula
|
 |
 |
|
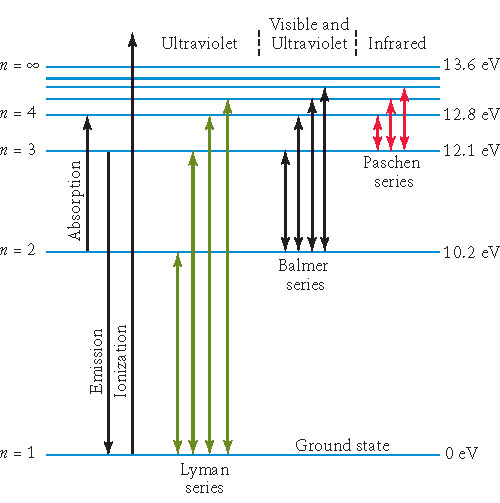
|
| c=3.00 x 108 m | h=6.63 x 10-34 J.s | kB=1.38 x 10-23 J/K | R = 1.10 x 107 m-1 | 1 eV = 1.60 x 10-19 J |
 |
 |
 |
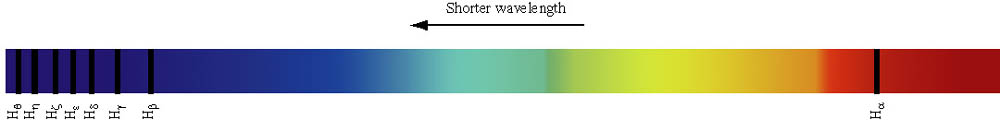
|
Waves in water, air ...
|
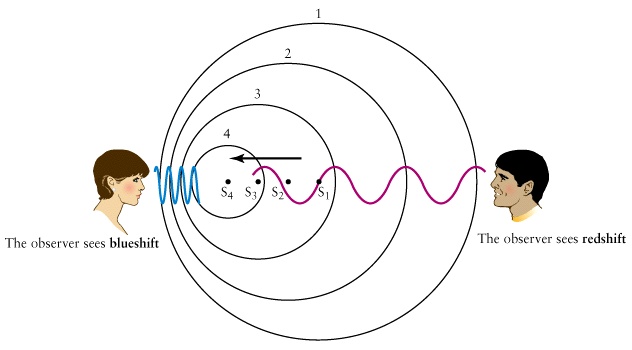 Useful animations 1,
2
Useful animations 1,
2
|
