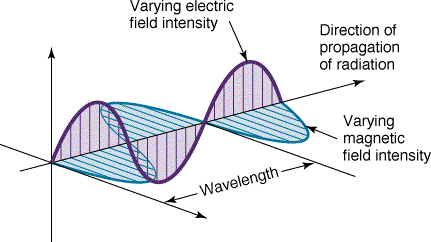
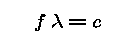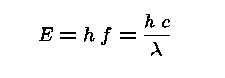For any type of wave (eg. sound, water, light)
- The wave travels at a constant speed = c.
- The distance between wavecrests is called the wavelength.
Wavelength is denoted with the Greek letter "lambda" (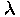 )
)
- The frequency is the number of times that the wave
oscillates in a second.
Frequency is denoted with the letter " f ".