J Pharm Pharmaceut Sci (www.ualberta.ca/~csps) 7(2):200-204, 2004
Simultaneous determination of vitamins C, E and β-carotene in human plasma by high-performance liquid chromatography with photodiode-array detection.
Bin Zhao, Su-Yin Tham, Jia Lu, Mui Hoon Lai, Lionel K.H. Lee, Shabbir M Moochhala1
Defence Medical and Environmental Research Institute, DSO National Laboratories, (DMERI @ DSO), Singapore, Republic of SingaporeReceived 03 November 2003, Revised 05 May 2004, Accepted 11 May 2004, Published 30June 2004
PDF Version
Abstract
PURPOSE: To develop a high-performance liquid chromatography (HPLC) method with photodiode-array ultraviolet detection for the simultaneous determination of vitamin C, vitamin E and b-carotene. METHODS: Following liquid-phase extraction from the human plasma samples, these three vitamins were successfully separated on the LiChrospher 100 RP-18 column (125 × 4 mm I.D.; particle size, 5 mm) at a flow-rate of 1.2 ml/min, with a mobile phase of methanol-acetonitrile-tetrahydrofuran (75: 20: 5, v/v/v). Results: The limits of quantitation were 100, 0.25 and 0.25 mg/ml for vitamin C, vitamin E and b-carotene respectively. The method is linear over the studied range of 0.25 to 5 g/ml for vitamin E and β-carotene and 100 to 5000 mg/ml for vitamin C. The extraction recoveries were greater than 83% for these three vitamins. The within day and between-day precision of the analysis did not exceed 15.3 and 16.2%, respectively. CONCLUSION: A suitable method to determine the concentration of vitamin C, vitamin E and β-carotene following oral administration of antioxidant supplement capsules to a healthy Chinese volunteer.
Introduction
Free radicals are highly reactive molecules that react with and damage cells throughout the body. They are suspected of causing cardiovascular disease, cancer, neurological disorders, cataracts, arthritis, aging and other conditions such as muscle damage and fatigue that could inhibit performance (1, 2). Antioxidants are molecules, which can safely interact with free radicals and terminate the chain reaction before vital molecules are damaged. There are several enzyme systems within the body that scavenge free radicals, whereby the principal micronutrient (vitamin) antioxidants are vitamin C, vitamin E and β-carotene. The body cannot manufacture these micronutrients so they must be supplied in the diet (2).
Many researchers believe that vitamin supplements can dramatically reduce free radical damage, prevent and delay the onset of chronic degenerative diseases, and possibly extend lifespan (3). Numerous epidemiological studies have demonstrated an association between higher intakes or higher blood concentrations of certain antioxidants and a lower incidence of certain degenerative diseases (4, 5). Clinical studies have shown that supplemental levels of antioxidant vitamins reduce an individual's risk for certain cancers and cardiovascular diseases (6, 7). Moreover, studies have shown that fruit and vegetable consumption (the major source of antioxidant nutrients) has a protective effect against cancer (8). Evidence also suggests that vitamins C, E and β-carotene supplementation have ergogenic or performance enhancing effects (9).
High-performance liquid chromatography (HPLC) combined with a UV-Vis detector is the most common method for identification and quantification of antioxidant vitamins in biological fluids. Several HPLC methods have been presented for the determination of vitamin C in serum or plasma (10, 11, 12, 18). The procedures for simultaneous measurement of vitamin E and β-carotene by HPLC have also been described (13, 14, 17). Recently, an isocratic liquid chromatographic method was reported for the simultaneous determination of vitamins C, E and β-carotene in pharmaceutical preparations (15). However, no reports for the simultaneous determination of these three antioxidant vitamins in human biological fluids were described. This is attributed to the extraction and reconstitution difficulties from biological fluids that are linked to their chemical properties (Vitamin C is water-soluble; Vitamin E and β-carotene are fat-soluble).
In this paper, we report a simple, sensitive and reliable isocratic HPLC assay for the simultaneous determination of antioxidant vitamins (vitamin C, vitamin E and β-carotene) in human plasma using photodiode-array detection. This method has been successfully employed to monitor plasma vitamin C, vitamin E and β-carotene levels following oral administration of antioxidant supplement capsules to a healthy Chinese volunteer.
Methods
Chemicals and materials:
Vitamin C (L-ascorbic acid, purity: 99%), vitamin E [(±)-alpha-tocopherol, purity: 95%) and β-carotene (purity: 97%) were obtained from Sigma Chemical Co. (St. Louis, MO, USA). Methanol, acetonitrile and tetrahydrofuran were obtained from Merck KGaA (Darmstadt, Germany). All solvents were of HPLC grade. All the reagents were used without further purification. Deionized water, purified by Milli Q system (Millipore, Milford, MA, USA), was used throughout the study. Stock solutions of vitamin E and β-carotene were prepared at 10 mg/ml in chloroform. Stock solution of vitamin C was prepared at 10 mg/ml in methanol. All stock solutions were protected from light and stored at -20ºC. The stock solutions were further diluted with methanol to give a series of working standards. The working solutions for spiking blank human plasma samples were prepared fresh daily.
Instrumentation:
The HPLC system (Waters 2690 Separation Module) consisted of a Waters 600E multisolvent delivery system pump, a Waters Ultra WISP 715 autoinjector, and a Waters 996 diode-array detection system set in a range of 200 - 400 nm (all from Waters, Milford, MA, USA). Vitamins C, E and beta-carotene were separated on the LiChrospher 100 RP-18 column (125 × 4 mm I.D.; particle size, 5 mm) from Merck KGaA (Darmstadt, Germany), with a mobile phase of methanol-acetonitrile-tetrahydrofuran (75: 20: 5, v/v/v) at a flow-rate of 1.2 ml/min.
Sample preparation:
Human plasma samples were spiked with concentrations ranging from 100 to 5000 μg/ml for vitamin C and from 0.25 to 5 μg/ml for vitamin E and b -carotene. Vitamin C in plasma was extracted as follows: plasma protein was precipitated with 60% methanol and 1mM EDTA. Plasma (100ml) was mixed with 400μl of 60% methanol/EDTA, incubated for 10min at 4ºC before centrifuging at 12,000 rpm for 8min. The clear phase was transferred to another polypropylene tube and evaporated to dryness under nitrogen. The dried extracts were dissolved in 100ml of methanol.
Vitamin E and b-carotene in plasma were extracted as follows: 100ml of plasma was deproteinized with 100ml of ethanol and was extracted with 600ml of chloroform. The extract was shaken for 5min before centrifuging. The organic layer was extracted and evaporated to dryness under nitrogen. The dried extracts were dissolved in 100ml of methanol. All reconstituted antioxidants were mixed together before injecting into the HPLC system.
Assay validation:
Samples were quantified using peak area of vitamin C, vitamin E and b-carotene. Standard calibration curves were constructed by spiked drug-free pooled human plasma with a known amount of vitamin C, vitamin E and b-carotene. These plasma standards were also used to determine the extraction recovery, within day and between-day precision (n = 5) of the method. The recoveries of vitamin C, vitamin E and b-carotene after liquid-phase extraction were calculated by comparing observed vitamins C, E and b-carotene peak areas in extracted plasma, to those of non-processed standard solutions. Limit of quantitation is based on the lowest concentration validated by the method.
Applicability:
One healthy non-smoking Chinese volunteer (male, aged 25 year) ingested three capsules of antioxidant supplement (Antioxidant Fuel, Twin Laboratories Inc., NY, USA) each day for 40 days. Each capsule contains 333 mg of vitamin C, 266 IU of vitamin E, 8,333 IU of b-carotene, 39 mg of dicalcium phosphate anhydrous, 33 g of sodium selenate, 10 mg of Co enzyme Q10, 66 mg of N-acetyl-L-cysteine, 33 mg of L-glutathione and 33 mg of alpha-lipoic acid. Venous blood samples were collected just before the first oral dose, and on the 15th, 30th, 33rd, 34th, 36th and 40th day of the study period. . The plasma was obtained immediately by centrifugation at 2000 g for 5 min and stored at -20ºC until analysis by HPLC.
Results and Discussion
In the current study, we developed a simple HPLC method for simultaneous determination of antioxidant vitamins (vitamins C, E and b-carotene) in human plasma. The solubility and polarity of the three analytes are vastly different, thus leading to the need for different grades of polar solvents to extract and constitute. After extraction, the final component reconstituents were mixed to enable a single injection into the HPLC. This method utilizes the photodiode-array detector for measuring three vitamins at various wavelengths in a single injection. The photodiode-array detector has an added advantage of effecting a rapid identification of UV-Vis spectra. In addition, peak purity of plasma samples as compared with standards can be monitored by this detection method.
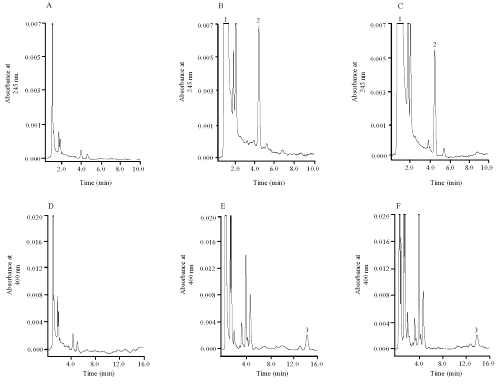
Representative chromatograms of blank and spiked human plasma samples are shown in Figure. 1.
Figure 1: HPLC chromatograms of blank human plasma (A) and (D), blank human plasma spiked with vitamin C, vitamin E with concentration of 2.5mg/ml and 2.5μg/ml respectively (B) and beta-carotene with concentration of 2.5mg/ml (E); and plasma sample from the subject (C) and (F). Peak 1: vitamin C, peak 2: vitamin E, and peak 3: beta-carotene. Chromatogram (A), (B) and (C) were detected at 245nm, whereas (D), (E) and (F) were detected at 400nm.
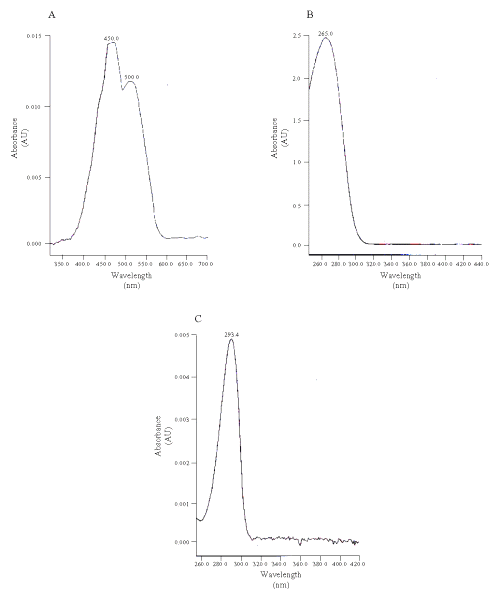
The wavelength for the detection of vitamins C and E was 245 nm (upper panels); and was switched to 400 nm for b-carotene (lower panels). Figure 2 shows the spectra of the compounds.
Figure 2: UV absorbance spectra of the standard (A) beta-carotene, (B) vitamin C and (C) vitamin E.
Under the described conditions, the retention times were 0.92, 4.1 and 13.7 min for vitamin C, vitamin E and b-carotene, respectively. The total run time was 15 min. clean chromatogram shows negligible interference from endogenous substances in plasma sample. Limits of quantitation were 100 mg/ml for vitamin C, 0.25 mg/ml for vitamin E and 0.25 mg/ml for b-carotene. Regression analysis was performed on the calibration curve in plasma. The calibration curves of the analytes obtained for five independent runs were linear within the studied range between 0.25 to 5 g/ml for vitamin E and b-carotene, and 100 to 5000 g/ml for vitamin C based on the peak area. The regression equations describing the calibration runs were vitamin C, y = 500000x + 88306 (R2 = 0.9918); vitamin E, y = 12486x + 7609 (R2 = 0.9781); and β-carotene, y = 45052x + 2844 (R2 = 0.9929), where y is the peak area of the antioxidants and x is the concentration. These studied ranges were found to be appropriate to cover the range of analyte concentration in plasma after oral administration of antioxidant supplement capsules to the healthy Chinese volunteer. The extraction recoveries of standards spiked in plasma samples were calculated. The recoveries of vitamin C, vitamin E and b-carotene within the concentration range of the assay were 83%, 100% and 91% respectively.
The vitamins under study are unstable and especially sensitive to light, heat, oxygen and peroxide (16). Therefore, special precautions have to be during the experiments. Stock solutions were tested four times from the time of their preparation up to 14 days and the results indicated that the stock solutions were stable within this period. However, the working solutions for spiking blank human plasma samples were freshly prepared from these stock solutions, and used immediately.
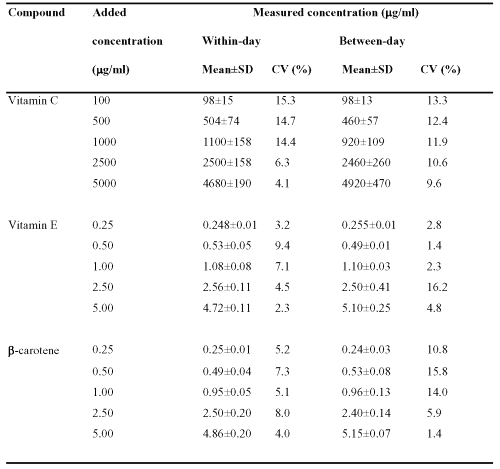
The within- and between-day variations (n = 5) of the method are given in Table 1.
Table 1: Within- and between-day assay variability (n = 5)
For each level, the repeatability and reproducibility criteria were clearly satisfactory. For the three analytes, within-day variability was lower than 15.3 % while between-day variability did not exceed 16.2 %. Within-day variability averaged 11.0 % for vitamin C, 5.3 % for vitamin E and 5.9 % for b-carotene. Mean between-day variability was 11.6 % for vitamin C, 5.5% for vitamin E and 9.6 % for b-carotene. To minimize analytical variability, analyte concentrations in human plasma were always derived according to the same day standard curve.
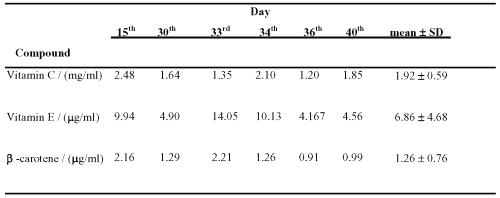
The applicability of the assay was demonstrated in plasma samples following oral administration of antioxidant supplement capsules to one healthy Chinese volunteer. After liquid-phase extraction, HPLC chromatogram was obtained (Figure. 1). The results of the assay are shown in Table 2.
Table 2: Blood concentration of antioxidants after ingestion of vitamin supplements at various time points.
The amount of vitamin C, vitamin E and b-carotene in plasma was above the limit of detection of the assay.
Conclusions
The proposed HPLC method, which combines photodiode-array detection, reaches the optimum performance in terms of selectivity, precision, and accuracy for antioxidant study in humans. The procedure employs a relatively simple liquid phase clean-up procedure for sample preparation. The efficiency is enhanced by the use of only one isocratic chromatographic elution that separates and quantifies the water-soluble vitamin C and fat-soluble vitamin E and b-carotene. This method can be utilized in studies that require monitoring of plasma vitamin C, vitamin E and b-carotene levels after oral administration of antioxidant supplement capsules to human subjects.
References
Fang, Y.Z., Yang, S. and Wu, G.Y., Free radicals, antioxidants, and nutrition. Nutrition, 18: 872-879, 2002.
Food and Nutrition Board, Institute of Medicine. Dietary Reference Intakes for Vitamin C, Vitamin E, Selenium, and Carotenoids, National Academy Press, Washington, D.C., USA, 2000.
Jacob, R.A. and Sotoudeh, G., Vitamin C function and status in chronic disease. Nutrition in Clinical Care 5: 66-74, 2002.
Engelhart, M.J., Geerlings, M.I., Ruitenberg, A., Van Swieten, J.C., Hofman, A., Witteman, J.C., and Breteler, M.M., Dietary intake of antioxidants and risk of alzheimer disease. JAMA 287: 3223-3229, 2002.
Lachance, P.A., Overview of key nutrients: micronutrient aspects. Nutrition Review 56: S34-39, 1998.
Fleshner N.E., Vitamin E and prostate cancer. Urologic Clinics of North America 29: 107-113, 2002.
Wijnen M.H., Roumen R.M., Vader H.L., and Goris R.J., A multiantioxidant supplementation reduces damage from ischaemia reperfusion in patients after lower torso ischaemia. A randomised trial. European Journal of Vascular and Endovascular Surgery, 23: 486-490, 2002.
Silalahi J., Anticancer and health protective properties of citrus fruit components. Asia Pacific Journal of Clinical Nutrition 11: 79-84, 2002.
Clarkson P.M., in: B.M. Marriott (Ed.), Nutritional Needs in Hot Environments:Applications for Military Personnel in Field Operations, National Academy Press,Washington, D.C., USA 1993.
Lyold L.L. Warner F.P., Kennedy J.F., and White C.A., Ion suppression reversed-phase high-performance liquid chromatography method for the separation of L-ascorbic acid in fresh fruit juice. Journal Chromatography 437: 447-452, 1988.
Liau L.S., Lee B.L., New A.L., and Ong C.N., Determination of plasma ascorbic acid by high-performance liquid chromatography with ultraviolet and electrochemical detection. Journal of Chromatography 612: 63-70, 1993.
Chung W.Y., Chung J.K. Szeto Y.T., Tomlinson B., and Benzie I.F., Plasma ascorbic acid: measurement, stability and clinical utility revisited. Clinical Biochemistry 34: 623-627, 2001.
Lee B.L., Chua S.C., Ong H.Y., and Ong C.N., High-performance liquid chromatographic method for routine determination on vitamin A and E and b-carotene in plasma. Journal of Chromatography 581: 41-47, 1992.
Mathiasson, L., Turner C., Berg H., Dahlberg L., Theobald A., Anklam E., Ginn R.,Sharman M., Ulberth F., and Gabernig R., Development of methods for the determination of vitamins A, E and beta-carotene in processed foods based on supercritical fluid extraction: a collaborative study. Food Additives and Contaminants 19: 632-646, 2002.
Paulo M.G.,.Cabral Marques H.M., Morais J.A.G., and Almeida A.J., An isocratic LC method for the simultaneous determination of vitamins A, C, E and b-carotene. Journal of Pharmaceutical and Biomedical Analysis 21: 399-406, 1999.
Reinhard E., in: L.M.L. Mollet (Ed.), Handbook of Food Analysis, Vol. 1, Marcel Dekker, New York, USA, 1996.
Palan P.R., Mikhail M.S., Goldberg G.L., Basu J., Runowicz C.D., Romney S.L. Plasma levels of beta-carotene, lycopene, canthaxanthin, retinol, and alpha- and tau-tocopherol in cervical intraepithelial neoplasia and cancer. Clin Cancer Res.2(1):181-5, 1996.
Motchnik P.A., Frei B., Ames B.N. Measurement of antioxidants in human blood plasma. Methods Enzymol. 234:269-79. 1994
Corresponding Author: Shabir M. Moochhala, DSO National Laboratories, (DMERI @ DSO), 27 Medical Drive, Singapore 117510, Republic of Singapore. MShabbir@dso.org.sg
Published by the Canadian Society for Pharmaceutical Sciences.
Copyright © 1998 by the Canadian Society for Pharmaceutical Sciences.
http://www.ualberta.ca/~csps