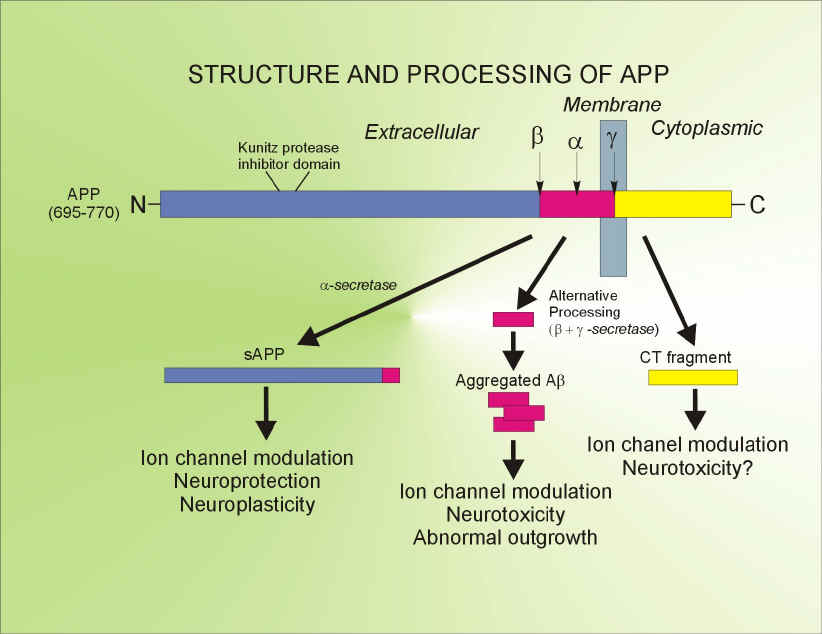
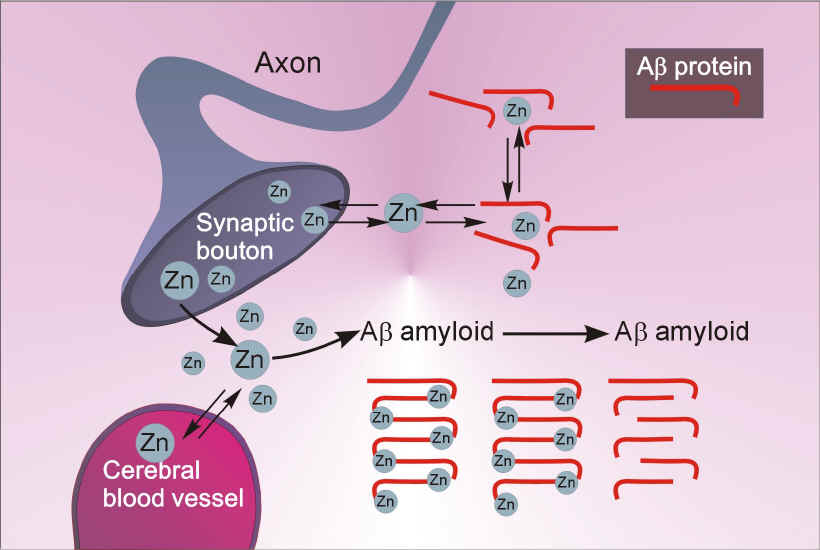
The second area of study is the diagonal band of Broca (DBB), a basal forebrain region where cholinergic neurons degenerate and die in Alzheimer's disease (AD). Although the precise mechanisms for the selective cell loss of cholinergic cells are not fully understood, certain neurotransmitters may produce uncontrolled excitation and eventual cell death of nerve cells in this region. We have previously focused our attention on studying the effects of neurotensin, vasopressin and zinc, which are substances that participate in memory and learning processes, and whose levels are altered in the brains of Alzheimer's patients. More recently, we have directed our attention to amyloid $-protein (A$), a 39-43 amino acid peptide, whose deposition in the brain is a primary pathological feature in AD. Currently we are investigating, at a cellular and molecular level, the mechanisms underlying the interactions between A$ with amylin and nicotinic acetylcholine receptors (nAChRs) of the rat cholinergic basal forebrain. Although the precise role of A$ in the genesis of Alzheimer’s disease (AD) is unresolved, available evidence indicates that A$ deposition and accumulation in the brain may initiate and/or contribute to the process of neurodegeneration. The loss of cholinergic basal forebrain neurons, which are amongst the most vulnerable neuronal populations in AD, results in severe memory and learning deficits, which are the hallmarks of clinical symptom complex of AD. There is considerable data to indicate that A$ influences cholinergic function in the brain and, under certain conditions, is neurotoxic to hippocampal and cortical neurons. In spite of its wide ranging effects in the CNS, there is as yet no “receptor” identified for A$. Human amylin, a pancreatic polypeptide, with limited primary sequence homology to A$, but possessing amyloidogenic properties, produces a profile of neurotoxicity in vitro that is strikingly similar to that produced by A$. Recently, it has been shown that A$ can compete for "4$2 and "7 nAChR binding sites in the brain and electrophysiological studies support A$-"7 receptor interactions in hippocampal neurons. These studies raise the intriguing possibility that amylin and nAChRs may serve as targets to mediate the effects of A$ in the brain. In support of this notion, our preliminary work indicates that (1) A$ inhibition of whole-cell currents in cholinergic basal forebrain neurons can be inhibited by AC 187, a specific antagonist for the amylin receptor (2) Single-channel studies in cholinergic basal forebrain neurons reveal that both A$ and nicotine activate an identical conductance of 76 pS, whose pharmacological profile indicates that it is coupled to the "4 nAChR subtype. These results, taken together, form the basis of the present application which hypothesizes that A$ acting via amylin and/or nAChRs regulates neurotransmission in the cholinergic basal forebrain and A$-mediated neurotoxicity may be expressed through the amylin receptor.
Current therapies for AD such as amyloid-based vaccines and secretase inhibitors show promise but are fraught with concerns over potential toxicity and interference with normal signalling pathways respectively. An identification and characterization of targets for the actions of A$ in the brain will serve not only to improve our understanding of the role that A$ may play in AD pathology, but also advance translational research in developing novel therapeutic drugs directed blocking the deleterious effects of A$ in the brain.

Home | Personnel | Publications | Lab Features | Funding | Contact | Links | U of Alberta |