Chemistry
Department
CHEM 261
Final
Exam
May 28,
2010
- Name the following compounds by the IUPAC
system. The name must indicate the stereochemistry of the compound. (12
points)
a.
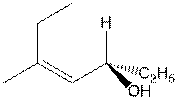
b.
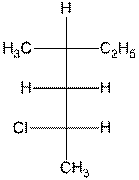
c.

- Complete the following partial Fischer structure of meso-3,4-dibromohexane:
(4 points)
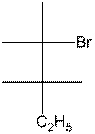
- Complete
the following partial structure of (E,5R)-5-bromo-3,6-dimethyl-3-heptene: (5 points)
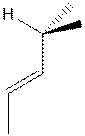
- Give
the structure(s) of the major organic products of five (5) of the following reactions:
a.

b.
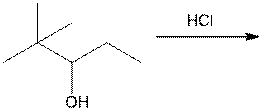
c.
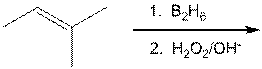
d.

e.
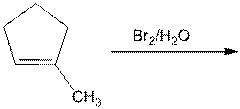
f.
![]()
- What
reagents would you use to effect five
(5) of the following conversions?
a.
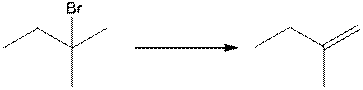
b.

c.
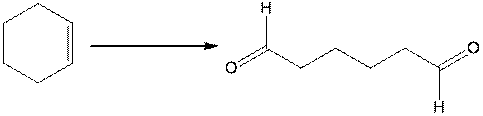
d.

e.
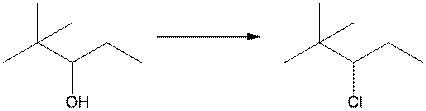
f.

- Provide
a synthetic pathway for the following transformations. Begin your synthesis with the indicated
starting material.
a.
(10 points)
![]()
b.
(8 points)

a.
Select
the member of each pair of compounds that will react faster by an SN2
mechanism: (2 points)
i.
CH3CH2CH2CH2NH2
or CH3CH2CH2CH2F
ii.
CH3CH2CH2CH2Cl
or CH3CH2CH2CH2Br
b. Which SN2 reaction of each pair would
you expect to take place more readily in a protic solvent? (2 points)
i.
CH3CH2CH2Cl +
(CH3)2S ® CH3CH2CH2S(CH3)2+
+ Cl-
or
CH3CH2CH2Cl + (CH3)2O
® CH3CH2CH2O(CH3)2+ + Cl-
ii.
CH3CH2CH2Cl
+ CH3CH2OH ® CH3CH2CH2OCH2CH3
+ Cl-
or
CH3CH2CH2Cl + CH3CH2O-
® CH3CH2CH2OCH2CH3
+ HCl
c. Which SN1 reaction of each pair would
you expect to take place more rapidly? : (2 points)
i.
(CH3)3CF + H2O
® (CH3)3COH + HF
or
(CH3)3CCl
+ H2O ® (CH3)3COH
+ HCl
ii.
(CH3)2CHBr + H2O ® (CH3)2CHOH + HBr
or
(CH3)3CBr
+ H2O ® (CH3)3COH
+ HBr
d.
1-Bromopropane reacts with an aqueous solution of
sodium hydroxide to form 1-propanol.
i.
Draw the most likely mechanism for this reaction. (Don’t forget the curved arrows). (3 points)
ii.
If potassium iodide is added to the reaction
mixture, the rate of formation of the 1-propanol is enhanced. Explain what
takes place. (3 points)
e.
3,3-Dimethyl-2-butanol reacts with HCl to form
2-chloro-2,3-dimethylbutane. Draw the most likely
mechanism for this reaction. (Don’t
forget the curved arrows). (8
points)
- When
the deuterium-labeled compound shown below is subjected to
dehydrohalogenation using sodium ethoxide in ethanol, the only alkene
product is 3-methylcyclohexene. The
product contains no deuterium.
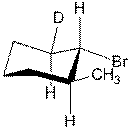
a.
What is a deuterium isotope effect? (2 points)
b.
Draw the most likely mechanism for this reaction
and explain why the deuterium is lost.
(Don’t forget the curved arrows). (9 points)
- 1-Methyl-1-vinylcyclopentane reacts with HCl to form
1-chloro-1,2-dimethylcyclohexane. Propose a mechanism to account for this
behaviour. (Don’t forget the curved arrows). (9 points)
- At the beginning of the biogenesis of
squalene isopentenyl pyrophosphate, CH2=C(CH3)CH2CH2OPP,
is enzymatically isomerized to dimethylallyl pyrophosphate, (CH3)2C=CHCH2OPP. These two compounds then react together
to yield geranyl pyrophosphate, (CH3)2C=CHCH2CH2(CH3)C=CHCH2OPP. Assuming that the weakly basic
pyrophosphate anion is, like the protonated hydroxyl group, a good leaving
group, R-OPP ® R+ + OPP- suggest a
mechanism by which geranyl pyrophosphate might be formed. (Don’t forget the curved arrows). (9 points)
- Caryophyllene, C15H24,
is a terpene which largely responsible for the odor associated with
cloves. Catalytic hydrogenation
gives a compound of formula C15H28.
a.
How
many units of unsaturation are present in caryophyllene? (1 point)
b.
How
many rings are present in caryophyllene?
(1 point)
Ozonolysis of caryophyllene gives
formaldehyde, H2C=O, and the following compound:
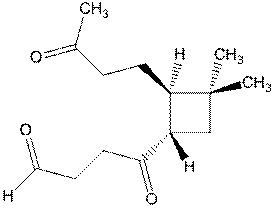
c.
How
many double bonds are present in caryophyllene?
(1 point)
Hydroboration of caryophyllene with one
equivalent of diborane followed by ozonolysis yields:
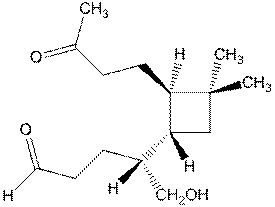
d.
Draw
the structure of caryophyllene. (4
points)