J Pharm Pharmaceut Sci (www.cspscanada.org) 9(2):165-168, 2006
Production and characterization of monoclonal antibodies against shope fibroma virus superoxide dismutase and glutathione-s-transferase
Soraya Shahhosseini, Sujatha Guttikonda, Pravin Bhatnagar, Mavanur R Suresh
Faculty of Pharmacy and Pharmaceutical Sciences, University of Alberta, Edmonton, AB, Canada.
Date accepted June 7, 2006; published June 14, 2006
Corresponding Author: Dr. M.R. Suresh, Phone: 1-780- 492-9233, Fax: 1-780-492-1217, Email: msuresh@pharmacy.ualberta.ca
ABSTRACT: PURPOSE: The superoxide dismutase (SOD) like proteins encoded by Leporipoxviruses play a role in regulating the redox status of infected cells. The biological function of these proteins is unclear. Why poxviruses encode these proteins are still unknown. Exploiting standard hybridoma techniques, we developed a monoclonal antibody (MAb) against shope fibroma virus superoxide dismutase (sfvSOD) to be used in diagnostics and as tools to understand the role of SOD-like proteins in pathogenesis. METHODS: Hybridoma cell fusion technology was used for production of MAbs. Balb/c mice were immunized with sfvSOD-GST fusion protein. Hybridoma clones were screened using indirect enzyme linked immunosorbent assay (ELISA). Specificity and reactivity of the MAbs were determined by Western blot analysis (WBA) and indirect ELISA. Protein G affinity chromatography was used for the purification of MAbs. RESULTS: Two stable hybridoma clones producing MAbs against the two domains of the fusion protein were obtained. The anti-GST (glutathione-s-transferase) and anti-sfvSOD MAbs were found to react specifically with GST and sfvSOD proteins respectively, in addition to the sfvSOD-GST fusion protein. Isotypes of these MAbs were identified as IgG2b heavy chain and k light chain.CONCLUSION: The anti-sfvSOD MAb (P115.SOD MAb) has been successfully used in studying the enzymatic and biochemical properties of a SOD homolog encoded by sfv. We also developed a strong anti-GST MAb which was also cloned and characterized P115.GST MAb. The anti-GST MAb might be useful in analyzing GST fusion proteins and in immunoaffinity chromatography purification of GST fusion proteins.
Introduction
The expression of S131R gene of Leporipoxvirus shope fibroma virus (sfv) leads to synthesis of homologs of cellular Cupro-Zinc SOD (Cu-Zn-SOD). The viral SOD homologs lack dismutase activity and potentially alter the redox state of infected cells. They reduce the activity of cellular Cu-Zn-SOD and affect the host antiviral defense. The mechanism of how the sfvSOD proteins might modulate the activity of cellular Cu-Zn-SOD is unknown (1-2). The role of viral SOD-like proteins are yet unclear and generating MAbs against these proteins can be useful as diagnostic tools as well as understanding their role in pathogenesis. Towards this objective, a MAb against sfvSOD protein was prepared. Here we describe the production, purification, and characterization of anti-sfvSOD MAb.
METHODS
Preparation of mouse monoclonal hybridoma cell line
The 6-8 week old female Balb/c mice were immunized intraperitoneally four times with fusion protein sfvSOD-GST at days 0, 14, 21, and 31 using complete and incomplete Freund’s adjuvant (Sigma) (30 mg / 200 ml) for the first and second immunizations respectively. Antigen in phosphate buffer saline (PBS) pH 7.3 (15 mg/ 200 ml) was used for the two other injections. The fusion protein was provided by Dr. David Evans, University of Guelph, Canada (2). Briefly, the recombinant sfvSOD was prepared by amplification of sfv S131R gene using the PCR and cloning in two steps in an E.Coli pGEX2T expression vector. The fusion protein was then produced in E.Coli bearing sfvSOD fused to GST. The recombinant protein was affinity purified and cleaved free of the GST domain using thrombin. The immune response to the antigen was assessed by measuring the titer of polyclonal antibody in mouse serum using ELISA. Three days prior to cell fusion, the mouse with high titer was boosted by surgical intrasplenic delivery of 30 mg of sfvSOD-GST fusion protein (3). The spleen cells were obtained 3 days later and fused with SP2/0 myeloma cell line at a ratio of 5:1 using 50% (w/v) polyethylene glycol (PEG) (Sigma) according to the technique previously described by Kohler and Milstein (4). The hybridoma cells were suspended in RPMI 1640 media (Invitrogen) supplemented with Penicillin Streptomycin L-Glutamine (PSG) (Sigma), Hypoxanthine Aminopterin Thymidine (HAT) (Sigma), and 20% fetal bovine serum (FBS) (Cansera). The cells were seeded in 96-well tissue culture plates and incubated in a humidified 37oC, 5% CO2 incubator for two weeks. Clones were cultured in Hypoxanthine Thymidine (HT) (Sigma) medium for further two weeks. Hybridoma cell lines producing specific antibodies were recloned by limiting dilution three times to ensure monoclonality and stability of the cell line.
Screening by indirect ELISA
Antibody secretion by hybridoma cells was detected by indirect ELISA. Briefly, 100 ml purified fusion protein (3 mg/ml) was used per well for coating flat bottomed 96 well plates (Nunc-Immuno MaxisorbTM plates, Nunc) overnight at 4oC. The wells were washed three times with PBS pH 7.3. To avoid nonspecific binding, the wells were incubated with 2% bovine serum albumin (BSA), for 2 h at room temperature (RT). After washing, the wells were incubated with 100 ml supernatant from each hybridoma clone for 2 h at RT. The wells were washed three times with PBS and the bound antibodies were detected using goat anti mouse IgG conjugated with horseradish peroxidase (GAM-HRPO) (Sigma) as secondary antibody at a 1:10, 000 dilution in 1% BSA for 1 h at RT. After final washing, 100 ml of 1:1 solution of 3,3¢,5,5¢- tetramethylbenzidine (TMB) and hydrogen peroxidase (H2O2) (KPL) were added to each well and incubated for 15 min at RT. The optical density was measured at 650 nm using an ELISA Vmax kinetic microplate reader (Molecular Devices Corp, California, USA). The clones showing ELISA values 5 times higher than the negative control were considered positive. Serum of un-immunized mouse and control antibody were used as negative control. RPMI media and positive sera from hyperimmunized mice were used as blank and positive control, respectively.
Western blot analysis for antigen binding
The specificities of the MAbs were evaluated by Western blot analysis (WBA). The sfvSOD-GST fusion protein and thrombin cleaved sfvSOD-GST fusion protein were electrophoresed on SDS-PAGE using 10% acrylamide gel and transferred onto nitrocellulose membrane (5) using mini trans-blot apparatus (Bio-Rad) following the manufacturer’s instructions. Non-specific binding on the membrane was blocked with 5% skim milk in PBS-T (0.1% Tween 20 in PBS pH 7.3) for 1 h. Membrane was washed five times with PBS-T, cut into strips, and incubated individually with different hybridoma clone supernatants for 2 h. After washing five times with PBS-T, strips were reacted with GAM-HRPO (1:10,000 in PBS pH 7.3) for 1 h at RT. Following a final washing step, binding of MAbs to antigen was detected by the blue bands of insoluble product when the strips were incubated with 1mg/ml 4-chloro-1-naphthol (Sigma) in PBS containing 0.1% H2O2. The reaction was stopped by washing with tap water. In another attempt, the binding of MAbs to antigen was detected using the Enhanced Chemiluminescence’s (ECL) substrate (Amersham). In the control experiments, antigen strip was incubated with control MAb or un-immunized mice sera.
Subclass determination of MAbs
The immunoglobulin subclass was determined using the mouse hybridoma isotyping reagents according to instructions from the manufacturer (Sigma). The light chain type was determined using Goat anti-mouse lambda (l) and kappa (k) horseradish peroxidase conjugate (Southern Biotech, USA).
Production and purification of MAbs
The hybridoma cell lines were cultured in a 175 cm2 tissue culture flask. Cultures were allowed to grow until the media turned yellow, and the supernatant was collected. A protein G affinity chromatography column (Sigma) was equilibrated with PBS pH 7.3, and crude culture fluid was loaded by pumping into the column. The column was washed with the PBS until the absorbance at 280 nm was close to base line and monoclonal IgG was eluted as 1 ml fractions with glycine-HCl, pH 2.8 and neutralized with 1 M Tris pH 9. The purified IgG fractions were pooled together and dialyzed against PBS pH 7.3 overnight.
RESULTS
Balb/c mice were immunized with sfvSOD-GST fusion protein. Following three booster immunizations with the antigen, the titer of antibody in mice sera was determined using indirect ELISA. All mice had developed antibody titers, detectable even at a 1:6000 dilution of the serum. The mouse with the highest antibody titer was further boosted intrasplenically and PEG based fusion was done to generate hybridomas. The ten antigen positive hybridomas obtained were cloned and recloned 3 times successively. Strong and stable hybridoma cell lines were grown in small scale. Based on ELISA and WBA results, two hybridoma cell lines producing specific MAbs against sfvSOD and GST were selected. Each hybridoma cell line produced about 10 mg immunoglobulin per liter of cell culture supernatant with a titer of 1:103. Isotypes of these MAbs were identified as IgG2b heavy chain and kappa-light chain.
The SDS-PAGE of sfvSOD-GST fusion protein showed a prominent band at molecular weight of 44 kDa, along with a minor doublet at approximately 30 kDa. After digestion with thrombin, the SDS-PAGE showed a band at 31 kDa for released GST domain plus ~18 kDa sfvSOD polypeptides (Fig.1). The proteins were transferred to nitrocellulose membrane and the specificity and reactivity of the MAbs against sfvSOD and GST proteins were determined by WBA. Several primary clones and reclones were tested by WBA to select the specific anti-GST and anti-sfvSOD MAbs. In WBA, MAbs recognized antigen under reducing conditions. The anti-GST and anti-SOD MAbs detected 31 kDa and ~18 kDa sfvSOD polypeptide bands, respectively (Fig.2).
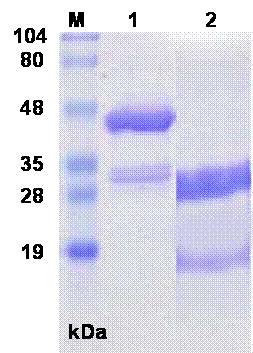
Fig.1. SDS-PAGE of sfvSOD-GST fusion protein and thrombin cleaved protein. The proteins were electrophoresed on 10% SDS-PAGE under reducing conditions. Lanes: M: Standard. MW. Marker, 1: sfvSOD-GST fusion protein 2: Thrombin cleaved sfvSOD-GST fusion protein.
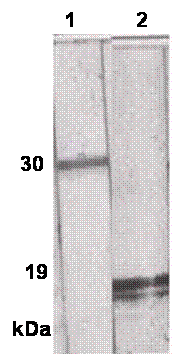
Fig.2. Reactivity of MAbs against thrombin cleaved fusion protein. The proteins were electrophoresed on 10% SDS-PAGE under reducing conditions and transferred onto nitrocellulose membrane. Membrane strips were individually incubated with P115.GST and P115.SOD MAbs. Lane 1: P115.GST MAb Lane 2: P115. SOD MAb.
DISCUSSION
Poxviruses encode a variety of proteins that serve to inhibit the activities of host antiviral defences. They might encode homologs of the Cu-Zn-SOD that play a key role in reactive oxygen species metabolism. Evans and associates (1-2) have suggested that Leporipoxvirus SOD homologs are catalytically inert decoy proteins that are designed to interfere in the proper metallation and activation of cellular Cu-Zn-SOD. This reaction might be advantageous for tumorigenic poxviruses, since higher levels of superoxide have been proposed to have anti-apoptotic and tumorigenic activity. The availability of the MAb allows the specific isolation of the SOD for molecular genetic comparisons of Cu-Zn-SOD.
In this study, anti-sfvSOD MAb (P115.SOD) was successfully developed using the GST fusion protein. In addition, we also developed a strong anti-GST MAb (P115.GST) which was also cloned and characterized. Indirect ELISA was used for screening of hybridoma clones. The specificity and reactivity of these two clones were tested using WBA and ELISA. The results presented in Fig.2 clearly demonstrate the specificity of the P115.GST and P115.sfvSOD MAbs for the GST and sfvSOD proteins, respectively. P115.SOD MAb was successfully used as a tool to characterize the enzymatic and biochemical properties of a SOD homolog encoded by sfv (1-2).
P115.GST MAb is useful in analyzing recombinant GST fusion proteins and in immuno-affinity chromatography for purification of GST-fusion proteins that fail to bind to glutathione beads, either due to denaturation or due to conformational change as an alternative to glutathione affinity chromatography (6).