J Pharm Pharmaceut Sci
(www.cspscanada.org) 9(1):133-139, 2006
Effects of sex hormones on
regulation of ABCG2 expression in the placental cell line BeWo.
Satoru Yasuda, Shirou Itagaki,
Takeshi Hirano and Ken Iseki*
Department of Clinical Pharmaceutics & Therapeutics, Graduate School of Pharmaceutical Sciences,
Hokkaido
University
, Sapporo, Japan
Received December 16, 2006, Revised, March
4, 2006,
accepted, March 15, 2006, published March
23, 2006.
PDF Version
__________________________________________________________________________________________
ABSTRACT: PURPOSE: The
aim of this study was to elucidate the effects of sex hormones that are
secreted during gestation from the placenta on ABCG2 mRNA and protein
expression levels by using the placental cell line BeWo. METHODS: We investigated the effects of estrogens (estrone,
17-β-estradiol and estriol) on the expression level of ABCG2 mRNA by
RT-PCR. The expression level of ABCG2 protein was analyzed by Western blot
analysis. We also investigated the
localization of ABCG2 in BeWo cells by Western blot analysis of the plasma
membrane fraction and by immunohistochemistry. RESULTS: It was found that all estrogens induce the expression of
ABCG2 mRNA in a concentration-dependent manner. Furthermore, Western blot
analysis showed that 17-β-estradiol induces the expression of ABCG2
protein. Western blot analysis of the plasma membrane fraction and
immunohistochemistry showed that ABCG2 localized on only the apical side of
BeWo cells and that 17-β-estradiol had no effect on the localization of
ABCG2. In addition, progesterone suppressed the induction of ABCG2 expression
by 17-β-estradiol at 1-10 µM. CONCLUSION:
The expression of ABCG2 in the placenta is regulated by estrogen and
progesterone during gestation.
Corresponding Author: Ken Iseki, Ph. D., Department of Clinical
Pharmaceutics & Therapeutics Graduate School of Pharmaceutical Sciences,
Hokkaido University Kita, Japan Tel/Fax:+81-11-706-3770
E-mail: ken-i@pharm.hokudai.ac.jp
INTRODUCTION
Placenta has been viewed as a protective barrier
and as a site for nutrient and waste exchange between the mother and fetus. A
group of transporters in the ATP-binding cassette (ABC) superfamily, such as
P-glycoprotein (P-gp) and multidrug resistance-associated proteins (MRPs), in
the placenta limit the entry of various potentially toxic drugs and xenobiotics
into the fetus. ABCG2 (also called breast cancer-resistant protein or
mitoxantrone-resistant protein) has also recently been found to be expressed in
the placenta at a level as high as the expression levels of ABC transporters [1]. It is known that ABCG2 transports
a variety of substrates, including anticancer drugs, doxorubicin, methotrexate
and SN-38, as well as endogenous hormones and nutrients [2-5].
Recently, there have
been some reports on regulation systems of ABCG2 expression. The structure and
characteristics of the ABCG2 promoter have been described, and the presence of
an estrogen response element in the ABCG2 promoter and estradiol-mediated
increase in ABCG2 mRNA expression in T47D:A18 cells have been reported [6]. However, it has been reported that
estrogen also down-regulates ABCG2 expression by a post-transcriptional
mechanism in MCF-7 cells [7]. It has
been shown that ABCG2 expression level in the human placenta, a major organ of
sex hormone secretion, changes with advance of gestational age [8]. Furthermore, we previously
reported that the expression level of ABCG2 decreases in the mid stage to end
stage of gestation in the rat and that progesterone might be involved in the
regulation of ABCG2 in the placenta (9).
It has been proposed
that ABCG2 plays an important role in the blood-placental barrier (BPB) for the
fetus. It is known that modulation of transporter expression and activity of
steroids is a key component in the placenta. Trophoblast cells express several
steroid receptors and are involved in many regulation systems [10-12]. However, the regulation system
of ABCG2 in the placenta is not known, and the effect of estrogen and the
combined effect of estrogen and progesterone on ABCG2 expression level have not
been investigated in detail. In this study, we used the human choriocarcinoma
cell line BeWo as a model of human trophoblast cells and investigated the
effects of sex hormones on regulation of ABCG2 in the placenta.
MATERIALS AND METHODS
Chemicals
Estrone, 17-β-estradiol, estriol and
progesterone were purchased from Wako (Osaka, Japan). All other reagents
were of the highest grade available and used without further purification.
Cell culture and hormone treatment
BeWo cells were obtained from Riken Cell Bank (Saitama, Japan). They were cultivated in nutrient mixture F-12
with Ham Kaighn's modification (Sigma Aldrich Japan, Tokyo) supplemented with
15% fetal bovine serum and 1% penicillin-streptomycin at 37°C under 95% air/5%
CO2. The cells were grown for 4-5 d, and after they reached
confluency they were washed with PBS and harvested by exposure to a
trypsin-EDTA solution and then passed into new flasks. Estrone,
17-β-estradiol, estriol and progesterone were dissolved in methanol and
added to the cells at various concentrations for a period of 72 h. Methanol
vehicle was used as a control.
RT-PCR analysis
Total RNA was prepared from BeWo cells using an
Isogen (Nippon Gene, Tokyo). Single-strand cDNA
was made from 2 µg total RNA by reverse transcription (RT) using an Omniscript
RT Kit. PCR was performed using Hot Star Taq PCR (QIAGEN) with ABCG2- and
GAPDH-specific primers through 33-40 cycles of 94°C for 30 s, 51°C for 1 min
and 72°C for 1 min. The primers specific to ABCG2 and GAPDH were designed on
the basis of sequence data in the GenBankTM database (accession no.:
NM_004827 and NM_002046, respectively). The sequences of the
specific primers were as follows: 5'-CAC CTT ATT GGC CTC AGG AA-3' (sense) and 5'-GAA ACA CTG GTT GGT CGT CA-3' (antisense) for hABCG2 and 5’-TGG
AAA TCC CAT CAC CAT CT-3’ (sense) and 5’-TTC TAG ACG GCA
GGT CAG GT-3’ (antisense) for GAPDH. The PCR products were subjected
to electrophoresis on a 1% agarose gel and then visualized by ethidium bromide
staining. ABCG2 mRNA data were expressed as ratios between the densitometric
values (Scion Image software) of each gene expression. The PCR products were
normalized to the amplified GAPDH, the internal reference gene.
Western
blot analysis
Total protein extracts were prepared from BeWo
cells. Cells were suspended in lysis buffer containing 1.0% Triton X-100, 0.1%
SDS, and 4.5 M urea. The suspension was left to stand for 5 min and sonicated
for 15 min at 4°C. Then it was centrifuged at 12,000 rpm for 15 min at 4°C, and
the protein concentration in the clear supernatant was determined by the method
of Lowry. The samples were denatured at 85°C for 3 min in loading buffer
containing 50 mM Tris-HCl, 2% SDS, 5% 2-mercaptoethanol, 10% glycerol, 0.002%
BPB, and 3.6 M urea and then separated on 4.5% stacking and 10% SDS
polyacrylamide gels. Proteins were transferred electrophoretically onto
nitrocellulose membranes (Trans-Blot; Bio-Rad) at 15 V for 90 min. The
membranes were blocked with PBS containing 0.05% Tween 20 (PBS/T) and 10%
non-fat dry milk for 1 h at room temperature. After being washed with PBS/T,
the membranes were incubated with monoclonal anti-breast cancer resistance
protein (BXP-21) (Sigma) (dilution of 1:200) or mouse anti-actin monoclonal
antibody (Chemicon) (dilution of 1:500) for 1 h at room temperature and then
washed with PBS/T (3 x 10 min). The membranes were subsequently incubated for 1
h at room temperature with horseradish peroxidase-conjugated goat anti-mouse
secondary antibody (Santa Cruz Biotechnology, Santa Cruz, CA) at a dilution of
1:4,000 and washed with PBS/T (3 x 10 min). The bands were visualized by
enhanced chemiluminescence according to the instructions of the manufacturer
(Amersham Biosciences Corp., Piscataway, NJ).
Preparation of membrane fraction
Vehicle- or 17-β-estradiol-treated BeWo
cells were prepared for the fractionation procedure. The plasma membrane
fraction was obtained by using a Plasma Membrane Protein Extraction Kit
(BioVision, Mountain view, CA) according to the
manufacturer’s instructions.
Immunocytochemistry
BeWo cells were fixed in 10% formaldehyde and
permeabilized in 0.1% Triton X-100 for 15 minutes. The cells were first
incubated in a blocking buffer (10% FBS in PBS) for 60 minutes. Then the cells
were incubated overnight at 4°C with monoclonal anti-breast cancer resistance
protein (dilution of 1:20). The cells were
subsequently incubated for 1 h at room temperature with rhodamine-conjugated
donkey anti-mouse secondary antibody (Santa Cruz Biotechnology) at a dilution
of 1:400. Nuclei were stained with DAPI and the cells were mounted in
VECTASHIELD Mounting Medium (Vector Laboratories, Burlingame, CA). The localization of
ABCG2 protein was visualized by using a confocal microscope (Zeiss LSM-510;
Carl Zeiss Inc., Thornwood, NY).
RESULTS
Effect of estrogen on expression level of ABCG2 mRNA
In the first part of this study, we investigated
whether expression of ABCG2 in BeWo cells is changed by estrogen treatment. In
all experiments, BeWo cells were treated with 0.01-10 µM estrogen in culture
medium for 72 h, because of no difference was observed in a shorter period
(data not shown). The expression level of ABCG2 mRNA was determined by semi-quantitative RT-PCR. We found that all estrogens,
estrone, 17-β-estradiol and estriol, induced expression of ABCG2 mRNA in a
concentration-dependent manner. The expression level of ABCG2 in cells treated
with 17-β-estradiol or estriol at 1-10 µM was 1.4-1.6-fold higher than
that in vehicle- treated cells (Figure 1).
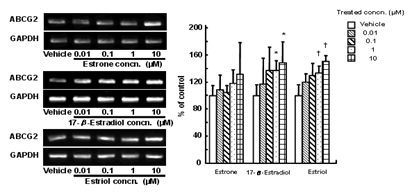
Figure 1: Effect of estrogen on expression of
ABCG2 in BeWo cells. Cells were treated with culture medium containing 0.01-10 µM estrone,
17-β-estradiol or estriol for 72 h. Methanol vehicle was used as a
control. ABCG2 mRNA levels were determined by semi-quantitative RT-PCR.
*P<0.05, +P<0.05 compared to each vehicle, using Student's unpaired t
test.
Effect of 17-β-estradiol on expression level of ABCG2
protein
It
is known that 17-β-estradiol is the strongest activator among the
endogenous estrogens for the estrogen receptor activation pathway and that estrogen is involved in
up-regulation of the expression of ABCG2 mRNA by genome reaction. We also examined the effect of 17-β-estradiol on
the expression level of ABCG2 protein in BeWo cells by Western blot analysis. A
single band for ABCG2 was observed at 60-70 kDa. The expression level of ABCG2
protein was increased by 2-4 fold in a 17-β-estradiol
concentration-dependent manner (Figure 2).
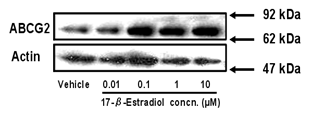
Figure 2: Effect of estradiol on expression
of ABCG2 protein in BeWo cells. Cells were treated with culture medium
containing 0.01-10 µM 17-β-estradiol for 72 h. Methanol vehicle was used
as a control. ABCG2 protein levels were determined by Western blot analysis.
Three µg of cell lysate was applied in each lane. Data shown are typical results
from three independent experiments.
Effect of
17-β-estradiol on localization of ABCG2 in BeWo cells
Figures
1 and 2 show the 17-β-estradiol-induced expression level of ABCG2.
However, it is known that 17-β-estradiol has an effect on retrieval of
some proteins from the plasma membrane to the intracellular domain. We
therefore investigated whether ABCG2 induced by 17-β-estradiol changes its
localization in BeWo cells. We prepared a plasma membrane fraction from BeWo
cells and determined the expression of ABCG2 by Western blot analysis (Figure
3).
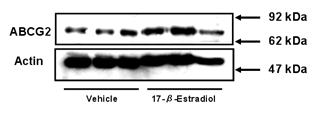
Figure 3: Expression level of ABCG2 protein
in plasma membrane fraction of BeWo cells. Cells were treated with culture
medium containing 10 µM 17-β-estradiol for 72 h. Methanol vehicle was used
as a control. Membrane fractions were prepared as described in Materials and Methods, and the
expression levels of ABCG2 were determined by Western blot analysis. One µg of
plasma membrane fraction was applied in each lane. Data shown are typical
results from three independent experiments.
The expression level of ABCG2 protein in
the plasma membrane fraction from 17-β-estradiol-treated cells was higher
than that in the membrane fraction from vehicle-treated cells. We also
performed immunocytochemistry to determine the localization of ABCG2. A red
signal (rhodamine, ABCG2) was observed at the plasma membrane (Figure 4A and
4C). In Z-sectioned
images, a red signal was observed at the
apical side of the cells and no red signal was observed at the same depth as
that of the blue signal (DAPI, nucleus), suggesting that ABCG2 protein was
expressed only in the apical membrane of BeWo cells (Figures 4B and 4D). There was no difference between the
localization of ABCG2 in vehicle-treated cells and that in
17-β-estradiol-treated cells.
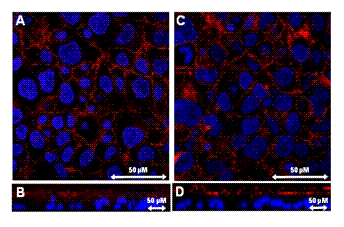
Figure 4: Immunohistochemistry of ABCG2 in
BeWo cells. Cells were treated with culture medium containing 10 µM
17-β-estradiol for 72 h (C, D). Methanol vehicle was used as a control (A,
B). Localization of ABCG2 was determined using an antibody against ABCG2 (red).
Nuclei were stained with DAPI (blue). B and D show Z-sectioned images.
Effect of progesterone on induction of the expression of
ABCG2 by 17-β-estradiol
Estrogen is not the only major hormone that the
placenta secretes; progesterone is also secreted from the placenta during
gestation. Moreover, we previously reported that the expression level of ABCG2
in BeWo cells is decreased by progesterone treatment. However, the combined
effect of estrogen and progesterone on ABCG2 expression has not been
investigated in detail. We examined the effect of progesterone on induction of
the expression of ABCG2 by 17-β-estradiol. We cultured BeWo cells in
medium containing 10 µM of 17-β-estradiol and various concentrations of
progesterone. Western blot analysis showed that progesterone had a suppressive
effect on the induction of ABCG2 expression by 17-β-estradiol. The level
of expression of ABCG2 induced by 17-β-estradiol was decreased to about
the same level as that in vehicle-treated cells in the presence of progesterone
at concentrations of 1-10 µM (Figure 5).
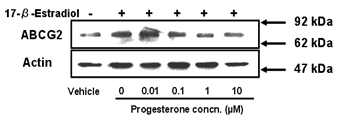
Figure 5: Combined effect of progesterone and
17-β-estradiol on expression of ABCG2 protein. Cells were treated with culture
medium containing 10 µM 17-β-estradiol and various concentrations of
progesterone for 72 h. Methanol vehicle was used as a control. Three µg of cell
lysate was applied in each lane. Data shown are typical results from three
independent experiments.
DISCUSSION
Syncytiotrophoblasts, which form the surface of
the placenta villi, play an essential role in restriction of drug import
through the BPB to the fetus. ABCG2 is expressed in the apical side of the
syncytiotrophoblast layer, a maternal-facing membrane in contact with the
maternal environment, and acts as an efflux pump of various compounds (13).
During gestation, ABCG2 in the placenta is thought to transport
estrogen-sulfate to the maternal side for protection of the fetus from
xenobiotics. Therefore, placental ABCG2 plays an important role in fetus growth
and development.
It
has recently been shown that ABCG2 has an estrogen response element in its
promoter region and that its expression in T47D:A18 cells is induced by
estrogen treatment [6]. On the other
hand, estrogen has been shown to induce post-transcriptional down-regulation of
ABCG2 in estrogen receptor-positive cell lines [7]. Furthermore, gender differences in the expression of ABCG2
have been observed in rats and mice. In the male rat kidney,
17-β-estradiol suppresses and testosterone induces expression of rABCG2
mRNA [14]. Therefore, it is known
that the expression of ABCG2 is regulated by steroids, but the regulation
system of ABCG2 expression in the placenta, which secretes various hormones,
has not been revealed in detail.
It
was shown in this study that the expression level of ABCG2 mRNA was increased
by estrone, 17-β-estradiol and estriol treatment in a
concentration-dependent manner (Figure 1).
It
was also shown that 17-β-estradiol treatment increased the expression
level of ABCG2 protein (Figure 2). This finding suggests that the expression of
ABCG2 is regulated by estrogen through the estrogen receptor-activated pathway
in the ABCG2 promoter region and that there is no post-transcriptional
down-regulation pathway by estrogen in the placenta.
17-β-estradiol
has various effects on some transporter regulation systems [15-17]. Multidrug resistance protein 2 in the sinusoidal membrane
of the rat liver was retrieved to the intracellular domain by
17-β-estradiol treatment [18].
Therefore, it is also possible that the systems by which ABCG2 is regulated by
estrogen in the placenta also a number of pathways. We investigated whether
ABCG2 protein induced by 17-β-estradiol changes its localization in BeWo
cells. Western blot analysis showed that the expression level of ABCG2 on the
plasma membrane was increased by 17-β-estradiol treatment (Figure 3).
Although only one µg of plasma membrane fraction showed a large amount of actin
as a house-keeping protein, this was because of the membrane skeleton was concentrated
at the process of the fractionation. We also found that ABCG2 was not expressed
on the intracellular domain and was expressed only on the apical membrane in
both vehicle-treated and 17-β-estradiol-treated cells, suggesting that its
localization was not changed by 17-β-estradiol treatment (Figure 4). These
results suggest that estrogen has only an inductive effect by genomic reaction
and no other effect on ABCG2 expression in the placenta.
We
showed that the expression level of ABCG2 was increased by estrogen in BeWo
cells, and it is known that estrogen secretion from the placenta increases with
advance of gestation [19]. On the
other hand, we previously reported that the expression level of ABCG2 decreases
from the mid stage to the end of gestation in the rat placenta [9]. Furthermore, it has been shown
that the expression level of ABCG2 in the human placenta decreases from
gestation days 90-120 to 60-90 [8].
Therefore, we hypothesized that the placenta has regulation systems other than
estrogen that suppress ABCG2 expression. We investigated the effect of
progesterone, which has been shown to down-regulate ABCG2 [9], on induction of ABCG2 expression by 17-β-estradiol. The
induction of ABCG2 expression by 17-β-estradiol was suppressed by combined
treatment with progesterone (Figure 5). It has been reported that progesterone
receptor (PR)-A and PR-B are expressed on JEG-3 cells [20], a subclone of BeWo cells. Several studies have shown that
PR-A acts as a dominant inhibitor of various other steroid receptors [21-22]. These findings suggest that
progesterone inhibited the effect of 17-β-estradiol at the
estrogen-estrogen receptor pathway by PR-A activation. However, the
concentration of each hormone used in this study is higher than the in vivo concentration during gestation [23]. It has also been reported that
folate deprivation down-regulates the expression level of ABCG2 in MCF-7 cells [24]. Since folate is an essential
nutrient for the fetus, folate might be involved in the regulation of ABCG2
expression in the placenta. Taken together, these findings indicate that
estrogen and progesterone secreted during gestation are involved in the
regulation system of ABCG2 in the placenta but that they are not the only
factors involved in the regulation of ABCG2 expression and that other
regulation systems and/or pathways exist. Further detailed investigation is
needed to reveal the relationship between the role and regulation mechanism of
ABCG2 in placenta.
In conclusion, estrogen induces the expression
of both mRNA and protein in BeWo cells. In addition, ABCG2 is localized at the
apical membrane of BeWo cells and 17-β-estradiol treatment has no effect
on its localization. These findings suggest that estrogen has no
post-transcriptional effect on the expression of ABCG2 but increases the
expression level of ABCG2 by genomic reaction in the placenta. Moreover,
progesterone suppresses the induction of ABCG2 expression by
17-β-estradiol. Taking these facts into consideration, sex hormones are
involved in the regulation of ABCG2 expression in the placenta during gestation.
REFERENCES
[1] Allikmets, R., Schriml,
L.M., Hutchinson, A., Romano-Spica, V. and Dean, M., A human placenta-specific
ATP-binding cassette gene (ABCP) on chromosome 4q22 that is involved in
multidrug resistance. Cancer Res,
58:5337-5339, 1998.
[2] Allen, J.D., Brinkhuis,
R.F., Wijnholds, J. and Schinkel, A.H. The mouse Bcrp1/Mxr/Abcp gene:
amplification and overexpression in cell lines selected for resistance to
topotecan, mitoxantrone, or doxorubicin. Cancer
Res, 59:4237-4241, 1999.
[3] Volk, E.L., Schneider, E.
Wild-type breast cancer resistance protein (BCRP/ABCG2) is a methotrexate
polyglutamate transporter. Cancer Res,
63:5538-5543, 2003.
[4] Imai, Y., Asada, S., Tsukahara, S., Ishikawa, E., Tsuruo, T.
and Sugimoto, Y., Breast cancer resistance protein exports sulfated estrogens
but not free estrogens. Mol Pharmacol,
64:610-618, 2003.
[5] Ifergan, I., Jansen, G. and Assaraf, Y.G., Cytoplasmic
Confinement of Breast Cancer Resistance Protein (BCRP/ABCG2) as a Novel
Mechanism of Adaptation to Short-Term Folate Deprivation. Mol Pharmacol, 67:1349-1359, 2005.
[6] Ee, P.L., Kamalakaran, S.,
Tonetti, D., He, X., Ross, D.D. and Beck, W.T., Identification of a novel
estrogen response element in the breast cancer resistance protein (ABCG2) gene.
Cancer Res, 64:1247-1251, 2004.
[7] Imai, Y., Ishikawa, E.,
Asada, S. and Sugimoto, Y., Estrogen-mediated post transcriptional
down-regulation of breast cancer resistance protein/ABCG2. Cancer Res, 65:596-604, 2005.
[8] Mathias, A.A., Hitti, J.
and Unadkat, J.D., P-glycoprotein and breast cancer resistance protein
expression in human placentae of various gestational ages. Am J Physiol Regul Integr Comp Physiol, 289:963-969, 2005.
[9] Yasuda, S., Itagaki, S.,
Hirano, T. and Iseki, K., Expression level of ABCG2 in the placenta decreases
from the mid stage to the end of gestation. Biosci
Biotechnol Biochem, 69:1871-1876, 2005.
[10] Patel, F.A., Funder, J.W.
and Challis, J.R., Mechanism of cortisol/progesterone antagonism in the
regulation of 15-hydroxyprostaglandin dehydrogenase activity and messenger
ribonucleic acid levels in human chorion and placental trophoblast cells at
term. J Clin Endocrinol Metab,
88:2922-2933, 2003.
[11] Hahn, T., Barth, S., Graf,
R., Engelmann, M., Beslagic, D., Reul, J.M., Holsboer, F., Dohr, G. and Desoye,
G., Placental glucose transporter expression is regulated by glucocorticoids. J Clin Endocrinol Metab, 84:1445-1452,
1999.
[12] Ni, X., Hou, Y., King,
B.R., Tang, X., Read, M.A., Smith, R. and Nicholson, R.C., Estrogen
receptor-mediated down-regulation of corticotropin-releasing hormone gene
expression is dependent on a cyclic adenosine 3',5'-monophosphate regulatory
element in human placental syncytiotrophoblast cells. J Clin Endocrinol Metab, 89:2312-2318, 2004.
[13] Kitano, T., Iizasa, H.,
Hwang, I.W., Hirose, Y., Morita, T., Maeda, T. and Nakashima, E., Conditionally
immortalized syncytiotrophoblast cell lines as new tools for study of the
blood-placenta barrier. Biol Pharm Bull,
27:753-759, 2004.
[14] Tanaka, Y., Slitt, A.L.,
Leazer, T.M., Maher, J.M. and Klaassen, C.D., Tissue distribution and hormonal
regulation of the breast cancer resistance protein (Bcrp/Abcg2) in rats and
mice. Biochem Biophys Res Commun,
326:181-187, 2005.
[15] Macheda, M.L., Rogers, S. and Best, J.D.,
Molecular and cellular regulation of glucose transporter (GLUT) proteins in
cancer. J Cell Physiol, 202:654-662, 2005.
[16] Kudo, N., Katakura, M.,
Sato, Y. and Kawashima, Y., Sex hormone-regulated renal transport of
perfluorooctanoic acid. Chem Biol
Interact, 139:301-316, 2002.
[17] Zampieri, L., Bianchi, P.,
Ruff, P. and Arbuthnot, P., Differential modulation by estradiol of
P-glycoprotein drug resistance protein expression in cultured MCF7 and T47D
breast cancer cells. Anticancer Res,
22:2253-2259, 2002.
[18] Mottino, A.D., Cao, J.,
Veggi, L.M., Crocenzi, F., Roma, M.G. and Vore, M., Altered localization and
activity of canalicular Mrp2 in estradiol-17beta-D-glucuronide-induced
cholestasis. Hepatology,
35:1409-1419, 2002.
[19] Casey, M.L., MacDonald,
P.C., Endocrine changes of pregnancy. In Williams Textbook of Endocrinology
1998 (9th Ed.) Wilson, J.D., Foster, D.W. Kronenberg, H.M. Philadelphia,
Saunders, pp 1259–1271, 1998.
[20] Cheng, K.W., Cheng, C.K.,
and Leung P.C., Differential role of PR-A and -B isoforms in transcription
regulation of human GnRH receptor gene. Mol
Endocrinol, 15:2078-2092, 2001.
[21] Wen, D.X., Xu, Y.F., Mais,
D.E., Goldman, M.E., and McDonnell, D.P., The A and B isoforms of the human progesterone
receptor operate through distinct signaling pathways within target cells. Mol Cell Biol, 14:8356-8364, 1994.
[22] Tung, L., Mohamed, M.K.,
Hoeffler, J.P., Takimoto, G.S., and Horwitz, K.B., Antagonist-occupied human
progesterone B-receptors activate transcription without binding to progesterone
response elements and are dominantly inhibited by A-receptors. Mol. Endocrinol, 7:1256-1265, 1993.
[23] Garland, H.O., Atherton,
J.C., Baylis, C., Morgan, M.R. and Milne, C.M., Hormone profiles for
progesterone, oestradiol, prolactin, plasma renin activity, aldosterone and
corticosterone during pregnancy and pseudopregnancy in two strains of rat:
correlation with renal studies. J
Endocrinol, 113:435-444, 1987.
[24] Ifergan, I., Shafran, A., Jansen, G., Hooijberg, J.H.,
Scheffer, G.L. and Assaraf, Y.G., Folate deprivation results in the loss of
breast cancer resistance protein (BCRP/ABCG2) expression. A role for BCRP in
cellular folate homeostasis. J Biol Chem,
279:25527-25534, 2004.
JPPS Contents
Published by the Canadian Society for
Pharmaceutical Sciences.
Copyright © 1998 by the Canadian
Society for Pharmaceutical Sciences.
CSPS Home | JPPS Home | Search | Subscribe to JPPS




