J Pharm Pharmaceut Sci
(www.cspscanada.org) 9(1):1-9, 2006
High-performance liquid chromatographic determination
of tanshinones in the
roots of Salvia miltiorrhiza and related
traditional Chinese medicinal preparations.
Ai-Hua Liu1, 2, Yan-Hua Lin1,
Min Yang1, Jiang-Hao Sun1, Hui Guo1, De-An Guo1,
2
1The State
Key Laboratory of Natural and Biomimetic Drugs, School
of Pharmaceutical Sciences,
Peking University,
Beijing P. R. China
2Shanghai Research Center
for TCM Modernization, Shanghai Institute of Materia Medica, Shanghai
Institutes for Biological Sciences, Chinese
Academy of Sciences, Shanghai, P. R. China
Received July 18, 2005; Revised November 21,
2005, Accepted November 23, 2005, Published December 19, 2005
PDF Version
﹛
ABSTRACT Purpose: This paper describes
a validated high-performance liquid chromatographic method to quantitate four
tanshinones as markers; dihydrotanshinone I, cryptotanshinone, tanshinone I and tanshinone IIA
for use in the quality control of the roots of Salvia
miltiorrhiza and its related traditional Chinese medicinal preparations.
Methods: Separation was achieved
using a Zorbax Extend C18 reserved-phase column (5µm, 250℅4.6mm) at 20 ∼C with a gradient mixture of deionized water and acetonitrile
at a flow rate of 1.2ml/min. Result: The
limits of quantitation were 0.13, 0.08, 0.06 and 0.05µg/ml for dihydrotanshinone
I, cryptotanshinone,
tanshinone I and tanshinone IIA, respectively. This method
provided good reproducibility and sensitivity for the quantification of four
tanshinones with overall RSD values for intra-day and inter-day precision and
accuracy better than 3.8% and higher than 94.9%, respectively. The recovery of
the method was 95.4-104.4% for all the tanshinones and showed good linearity
(r>0.9998) over a relatively wide concentration range. Conclusions: This assay was successfully applied to the
determination of four tanshinones in the roots of Salvia
miltiorrhiza and its related traditional Chinese medicinal preparations.
The results indicated that the HPLC assay could be readily utilized as a
quality control method for the roots of Salvia miltiorrhiza
and its related traditional Chinese medicinal preparations.
﹛
INTRODUCTION
The roots of Salvia miltiorrhiza (Danshen), a
commonly used herbal medicine in China, are widely used to treat
coronary heart disease, cerebrovascular disease, hepatitis, hepatocirrhosis and
chronic renal failure (1-4). Due to its purported biological activity and fewer
side effects as confirmed by pharmacological investigations and clinical use,
many traditional Chinese medicinal preparations (TCMPs) containing Danshen, such
as Fufang Danshen tablets (FDT), Compound Danshen Dripping pills (CDDP),
Danshen injection (DSI) and Xiangdan injection (XDI), have been developed.
These TCMPs are mainly used to treat coronary heart disease, heart-stroke, and
associated cerebrovascular and cardiovascular diseases (5-9).
To
ensure clinical efficacy, quality control of Salvia miltiorrhiza and its
related TCMPs is critical. Since Danshen is the major component of FDT, CDDP,
DSI and XDI, quality control of Danshen content is thus essential to guarantee
the quality of these TCMPs. Tanshinones are hydrophobic components isolated
from Danshen. It has been reported that tanshinones possess anti-toxin
properties, modulate immunological diseases, dilate coronary arteries, increase
coronary flow and protect the myocardium against ischaemia (10-13). In
addition, tanshinones have attracted particular attention because they have exhibited
significant antibacterial, anti-dermatophytic, anti-neoplastic and
anti-platelet aggregation activities (14-16).
In
the Pharmacopoeia of the People*s Republic of China (2005 Edition) (1), quality
control of Danshen and FDT has relied mainly on the determination of tanshinone
IIA. A number of studies on
the quantitation of tanshinones in Danshen and its related TCMPs by thin layer
chromatography (TLC), spectrophotometry or HPLC have been reported. However, only
a few tanshinones were determined in these papers (17-21). According to the
literature (22) and our study, dihydrotanshinone I, cryptotanshinone, tanshinone I and tanshinone IIA
(Fig. 1) are the main tanshinones contained in Danshen and its related TCMPs.
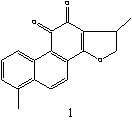
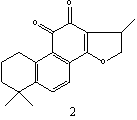
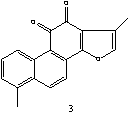
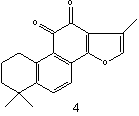
Figure 1: The structures of selected tanshinones: 1,
dihydrotanshinon I; 2, cryptotanshinone; 3,
tanshinone I; 4, tanshinone IIA
Therefore
simultaneous determination of these four tanshinones could, to some extent,
reflect the overall quantity of Danshen and its related TCMPs.
In this report, we
describe a simple, rapid and accurate method of analysis using reversed-phase HPLC
(RP-HPLC) to simultaneously determine dihydrotanshinone I, cryptotanshinone, tanshinone I and tanshinone IIA
in Danshen and in four TCMP samples.
﹛
MATERIALS AND METHODS
Chemicals and Materials
HPLC grade acetonitrile and methanol (E. Merck, Darmstadt, Germany)
were used for the HPLC analysis. Deionized water was purified by a Milli-Q
system (Millipore, Bedford,
MA, USA).
Phosphoric acid and chloroform were of analytical grade from Beijing Beihua
Fine Chemicals Co., Ltd. (Beijing,
China). The
roots of Salvia miltiorrhiza and TCMPs samples were purchased from
drugstores around China
(Table 4).
Dihydrotanshinone
I [1], cryptotanshinone [2],
tanshinone I [3] and tanshinone IIA [4] were purchased from the National Institute for Control of
Biological and Pharmaceutical Products of China, and their purity was over 98%
by HPLC analysis.
Apparatus and chromatographic conditions
An Agilent 1100 HPLC system (Agilent Technologies, Palo
Alto, CA, USA) comprising a quaternary solvent delivery system, an on-line
degasser, an autosampler, a column temperature controller and photodiode array
detector coupled with an analytical workstation was used. The column configuration
was an Agilent Zorbax Extend C18 reserved-phase column (5µm, 250℅4.6 mm) with an Agilent Zorbax Extend C18
guard column (5µm, 10℅4.6mm).
The sample injection volume was 10 米l.
The
detection wavelength was set at 270nm, the flow rate was 1.2ml/min and the
column temperature was maintained at 20 ∼C. The mobile phase consisted of A (deionized water)
and B (acetonitrile). Gradient elution was as follows: Initially 45% B at 0 min,
linearly increasing to 60% at 3 min, maintaining 60% B from 3 min to 14 min,
linearly increasing B to 80% at 15 min and then linearly increasing B to 82% at
20 min until the end of the run. After each analysis, 45% mobile phase B was pumped
and maintained for 10 min to re-equilibrate the system for baseline stability. An
SB 3500 ultrasonic generator (50 KHz, 350 W) from Shanghai Branson Ultrasonics
Co. Ltd (Shanghai, China) was used to aid the extraction
of tanshinones from the samples.
Table 1: Calibration
curves of tanshinones.
|
Analyte
|
Retention Time (min)
|
Standard curve
|
r 2
|
Test range
(µg ml-1)
|
LOD
(µg ml-1)
|
LOQ
(µg ml-1)
|
|
|
|
Dihydrotanshinone I
|
8.02
|
Y=24.321x+6.525
|
0.9998
|
1.4-64.1
|
0.05
|
0.13
|
|
Cryptotanshinone
|
11.95
|
Y=26.835x+11.065
|
0.9998
|
6.9-171.9
|
0.03
|
0.08
|
|
Tanshinone
I
|
13.23
|
Y=30.049x+0.850
|
0.9998
|
4.1-82.70
|
0.03
|
0.06
|
|
Tanshinone
II A
|
18.13
|
Y=41.220x+23.234
|
0.9999
|
30.86-185.14
|
0.02
|
0.05
|
Y: peak area;
x: concentration of analyte (µg ml-1)
An
FW100 pulverizer (24000 rpm/min, 460 W) from Tianjin City Taisite Instrument
Co. Ltd (Tianjin, China) was used to comminute the Danshen.
Quantitative filter paper (No.201, 9 cm)
supplied by Hangzhou Fuyang Special Industry Co., Ltd (Hangzhou, China) and the
membrane filters (13 mm,
0.45 米m) were obtained from Tianjin Tengda Filtration Instrument Co., Ltd
(Tianjin, China).
Preparation of calibration standard
solutions
A methanol stock solution containing compounds 1, 2,
3 and 4 was prepared and diluted to the appropriate concentration range
(Table 1) for the establishment of calibration curves. Each calibration curve
was analyzed three times with six different concentrations using the same HPLC
conditions as described above.
Sample preparation
The dried roots of Salvia miltiorrhiza were sheared
to be about 1cm in length
and then comminuted by pulverizer. After the coating was removed by abrasive
cloth, FDT and CDDP were ground in a mortar. Each solid sample (100 mesh, 0.300g) was suspended in methanol: chloroform
(7:3, v/v, 10 ml) and sonicated for 0.5h. After filtration, the filtrate was
transferred to a 25 ml volumetric flask and evaporated to dryness at 28 ∼C. The evaporated residue was
dissolved in methanol and made up to volume in a 10 ml volumetric flask. One
milliliter of either DSI or XDI liquid sample was diluted to 6 ml with
deionized water. The solutions were filtered through a membrane filter (0.45 米m)
and then injected into the HPLC.
Quality
control samples
On different days, QC samples containing the relevant
reference compounds (low, medium and high concentrations) were used to verify
the calibration curve. The calibration curve could be used on subsequent days when
the coefficient of variability (CV) of the QC samples was less than 2%, failing
which a new calibration curve would be established.
Precision and accuracy
The measurements of intra-day and inter-day variability were
utilized to determine the precision of the method. Three different
concentrations (low, middle and high) of the four standards were prepared. The
relative standard deviation (RSD) was used as a measure of precision. The intra-day variability was examined within one day (n=5) and
inter-day variability was
determined in triplicate on 3 separate days. Recovery experiments were
carried out by spiking FDT (0.25g,
brown powder ) with different amounts (low, medium and high) of authentic
standards with known contents of marker compounds and the samples were treated according
to the sample preparation procedure.
Limits of detection (LOD) & limits of
quantitation (LOQ)
LOD is defined as the lowest
amount of analyte which can be detected. It is formally defined as X-XB=3SB,
where X is the signal from the sample, XB is the signal from the
analytical blank and SB is the SD of the reading for the analytical
blank (26). LOQ is the lowest amount of an analyte which can be quantitated and
similar to LOD, LOQ is formally defined as X-XB=10SB
﹛
RESULTS
AND DISCUSSION
Optimization of Extraction Procedure and Chromatographic
Conditions
In
order to achieve quantitative extraction, variables involved in the procedure
such as solvent, extraction method and extraction time were optimized. A series
of solvents such as methanol (MeOH), methanol:chloroform (CHCl3)
(7:3, 5:5, 3:7, v/v) and CHCl3 were tested as the extraction solvents
of tanshinones (23-25). The best solvent was found to be a mixture of MeOH: CHCl3
(7: 3, v/v) (Table 2), which allowed extraction
of all the tanshinones in high yield. MeOH or CHCl3 alone were not
efficient for the complete extraction of the four compounds of interest.
Table 2: Comparison
of different extraction solvents.
|
Extraction Solvent
|
The peak area
of tanshinones
|
|
1
|
2
|
3
|
4
|
|
|
|
|
|
|
|
MeOH :CHCl3=7:3
|
287.7
|
539.0
|
863.1
|
2747.3
|
|
MeOH :CHCl3=5:5
|
165.9
|
324.3
|
504.7
|
1655.2
|
|
MeOH :CHCl3=3:7
|
140.7
|
276.8
|
433.6
|
1426.8
|
|
CHCl3
|
177.3
|
127.0
|
418.2
|
826.7
|
The ultrasonic treatment procedure was found to be the best
extraction method for the tanshinones. In order to investigate extraction time,
powdered FDT (0.300g) samples
were extracted with 10 ml MeOH: CHCl3 (7:3, v/v) for 10, 20, 30, 45,
60 min, respectively. The results suggested that all the tanshinones were
almost completely extracted within 30 min (Table 3).
Table 3: Comparison of extraction time.
|
Extraction Time
|
The peak area of tanshinones
|
|
1
|
2
|
3
|
4
|
|
|
|
|
|
|
|
20 min
|
464.4
|
1752.0
|
1012.9
|
2459.7
|
|
30 min
|
452.9
|
1811.2
|
1071.3
|
2522.4
|
|
45 min
|
464.8
|
1797.8
|
1063.0
|
2527.8
|
|
60 min
|
436.9
|
1778.3
|
1052.5
|
2457.9
|
Therefore,
the optimal extraction method of tanshinones was ultrasonic extraction with
10ml of the mixture of MeOH: CHCl3 (7:3, v/v) for 30 min.
A
good separation is assumed when the analyzed peaks are baseline separated
within a short analysis time. To obtain chromatograms with good separation, various
stationary phases, mobile phases, column temperatures, detection wavelengths
and flow rates were investigated. For the assay of tanshinones in Danshen and
its related TCMPs, a Zorbax Extend C18 was found to be better than BDS-Hypersil
C18, YMC-Pack ODS-A C18 or Luna C18. Various mixtures of water/acetonitrile or
water/methanol were used as mobile phase and the results indicated that
water/acetonitrile system was better than that of water/methanol. Due to the
similar retention behaviors of compounds 2
and 3, the mobile phase B (acetonitrile)
was maintained at 60% from 3 min to 14 min to achieve a baseline separation of
these two compounds. It was also found that the best separation was achieved
when the column temperature was kept at 20 ∼C
using a flow rate of 1.2ml/min and detection wavelength of 270 nm.
Linearity and range
Figure 2 shows a typical chromatogram of four tanshinones, 1, 2,
3 and 4 with retention times 8.02, 11.95, 13.23 and 18.13 min,
respectively.
﹛
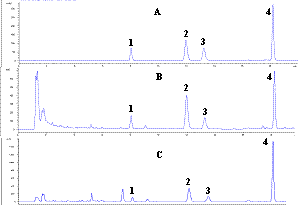
﹛
Fig. 2: Representative
HPLC chromatograms of (A) standard solution at medium concentration, (B) FDT (Xianzhi,Liaoning, China), (C) Danshen (Shanxi, China) 1,
dihydrotanshinon I; 2, cryptotanshinone; 3,
tanshinone I; 4, tanshinone II A.
﹛﹛
Table 4: Intra-and
inter-day variability.
|
Concentration (µg ml-1)
|
Inter-day
(n=9)
|
Intra-day
(n=5)
|
|
Found
|
RSDa (%)
|
Accuracyb
(%)
|
Found
|
RSD (%)
|
Accuracy
(%)
|
|
Dihydrotanshinone I
5.83
34.95
58.25
|
|
|
|
|
|
|
|
5.69㊣0.19
|
3.3
|
97.6
|
5.53㊣0.05
|
0.85
|
94.9
|
|
13.00㊣0.17
|
1.3
|
104.0
|
35.10㊣0.06
|
0.18
|
100.4
|
|
60.49㊣0.73
|
1.2
|
103.8
|
60.20㊣0.63
|
1.04
|
102.9
|
|
Cryptotanshinone
13.75
82.50
158.13
|
|
|
|
|
|
|
|
14.04㊣0.53
|
3.8
|
102.1
|
13.24㊣0.02
|
0.12
|
96.3
|
|
85.3㊣1.71
|
2.0
|
96.7
|
83.22㊣0.39
|
0.47
|
100.9
|
|
165.75㊣1.85
|
1.1
|
104.8
|
164.81㊣0.35
|
0.21
|
104.2
|
|
Tanshinone I
8.27
49.60
74.40
|
|
|
|
|
|
|
|
8.15㊣0.16
|
2.0
|
98.5
|
8.03㊣0.02
|
0.26
|
97.1
|
|
50.05㊣1.4
|
2.7
|
100.9
|
49.53㊣0.31
|
0.63
|
99.9
|
|
75.49㊣1.5
|
2.0
|
101.5
|
75.94㊣0.23
|
0.30
|
102.1
|
|
Tanshinone II A
38.6
92.6
177.4
|
|
|
|
|
|
|
|
37.04㊣1.04
|
1.0
|
95.9
|
37.23㊣0.07
|
0.18
|
96.4
|
|
89.44㊣2.01
|
2.3
|
96.6
|
90.71㊣0.22
|
0.24
|
98.0
|
|
172.31㊣4.70
|
2.7
|
97.1
|
178.90㊣0.45
|
0.25
|
100.8
|
aRSD(%) =
(SD/mean)℅100
bRecovery(%) =
(mean of measured concentration/spiked concentration)℅100
Table 5: Recoveries
of the four tanshinones (n=4).
tanshinone spiked (µg ml-1)
|
|
|
|
|
Dihydrotanshinone I
3.0
36.2
48.6
|
|
|
|
|
2.94㊣0.10
|
3.3
|
98.1
|
|
35.41㊣0.57
|
1.6
|
97.8
|
|
48.37㊣1.35
|
2.8
|
98.8
|
|
Cryptotanshinone
8.8
96.8
142.1
|
|
|
|
|
9.07㊣0.13
|
1.5
|
103.1
|
|
95.07㊣1.38
|
1.5
|
98.2
|
|
138.28㊣2.35
|
1.7
|
97.3
|
|
Tanshinone I
8.9
48.9
63.4
|
|
|
|
|
9.24㊣0.31
|
3.4
|
103.9
|
|
47.53㊣1.5
|
3.2
|
97.2
|
|
60.45㊣3.5
|
5.8
|
95.4
|
|
Tanshinone IIA
38.5
84.7
126.6
|
|
|
|
|
40.20㊣2.39
|
5.9
|
104.4
|
|
81.13㊣2.81
|
3.5
|
95.8
|
|
117.26㊣4.0
|
3.4
|
95.6
|
aRSD(%) =
(SD/mean)℅100, bRecovery(%) = (mean of measured concentration/spiked
concentration)℅100
﹛
The
four peaks in the samples were monitored in the UV range from 200-400nm with a
DAD-detector and assessment of peak purity showed peak homogeneity thereby indicating
the specificity of the method. Linear ranges and correlation co-efficients are
depicted in Table 1. All the standard compounds showed good linearity (r>0.9998)
in a relatively wide concentration range.
System suitability
The
intra-day precision is shown in Table 4 where the RSDs ranged from 0.12% to 1.04%.
The inter-day precision was determined from nine determinations over 3 separate
days for each concentration and the results were in the range of 1.00%-3.80%. The
recovery of the four standards ranged from 95.4% to 104.4% (Table 5).
Stability
testing was performed on a sample solution left on the bench and was analyzed
every 12 h over 3 days at room temperature. The analytes were found to be relatively
stable over 72 h (RSD<5%).
LOD and LOQ
The LODs of the four tanshinones were 0.05, 0.03, 0.03 and 0.02 mg ml−1 and the LOQs were 0.13, 0.08, 0.06 and 0.05 mg ml−1, for 1, 2,
3 and 4, respectively (Table 1).
Sample analysis
Forty samples were prepared as previously described. A
volume of 10 ml of
filtered solution of each sample was injected into the instrument and each
sample was determined in triplicate. Peaks in the chromatograms were identified
by comparing their retention times and on-line UV spectra with those of the reference
standards.
The method was successfully
applied to determinate four tanshinones in Danshen and in four Danshen-containing
TCMPs, FDT, CDDP, XDI and DSI, respectively. The contents of the four major
tanshinones in 40 samples are shown in
Table 6. From Table 6, it is obvious
that the quality of fourteen Danshen samples from different locations varied quite
drastically and could account for uncertain and unstable curative effects of
the TCMPs. Furthermore, it can be seen that the contents in four of the TCMPs
also varied markedly and are hardly detectable in DSI and XDI, low in content in
CDDP and high in Danshen and FDT samples. The reason for the variation might be
due to different processing procedures of Danshen during the manufacturing
process. It is also obvious from Table 6 that the contents of the tanshinones
in FDT varied greatly amongst the different manufacturers. The variation of
contents may be due to different quality of the raw material, the difference of
production procedure, storage and transportation, etc., amongst others.
In
the Pharmacopoeia of People*s Republic of China (2005 Edition), tanshinone IIA is claimed to be present in not
less than 0.2% of Danshen crude drug and 200米g/tablet for FDT. In our 14
samples of Danshen crude drugs analyzed, there are 11 samples (N0s.21, 22, 23
and 27-34) that meet the specified standard by Chinese Pharmacopoeia, while all
FDT samples determined meet this standard. Considering the purported medicinal
properties of tanshinone I,
crytotanshinone and dihydrotanshinone I,
it is recommended that not only tanshinone IIA but also tanshinone I,
crytotanshinone and dihydrotanshinoneI
should be determined for use as quality control markers for Danshen crude drug
and TCMPs containing Danshen.
﹛
CONCLUSIONS
This paper
describes a simple, accurate and precise method for the simultaneous
determination of the four major tanshinones in Danshen, FDT, CDDP, DSI and XDI.
The data obtained from 40 samples reveal that the contents of tanshinones in Danshen
and FDT varied considerably. The contents are very low in CDDP and almost
undetectable in DSI and XDI. The current HPLC method has thus been shown to be
suitable for use as a method for the quality control of Salvia miltiorrhiza and its
related TCMPs to assure their clinical efficacy.
﹛
Table 6: Contents
of tanshinones in samples of Danshen
and its related TCMPs.
|
NO
|
Batch Number or harvest timeh
|
Collection Province
|
Contentf(n=3)
|
|
Tanshinone IIA
|
Tanshinone I
|
Cryptotanshinone
|
Dihydrotanshinone I
|
|
|
|
|
|
|
|
|
|
2 a
|
31207
|
Beijing
|
531.3㊣9.0
|
201.5㊣9.3
|
528.5㊣21.4
|
123.0㊣6.1
|
|
3 a
|
40703
|
Shenzhen
|
534.6㊣6.3
|
144.3㊣6.7
|
291.6㊣5.2
|
93.9㊣3.2
|
|
4 a
|
30113
|
Guangxi
|
534.5㊣21.2
|
87.2㊣3.3
|
192.6㊣3.6
|
42.5㊣3.4
|
|
5 a
|
40210
|
Henan
|
714.4㊣18.6
|
477.4㊣3.4
|
768.7㊣6.2
|
270.3㊣21.6
|
|
6 a
|
9919
|
Hebei
|
411.6㊣6.7
|
210.5㊣12.7
|
273.5㊣3.1
|
84.2㊣3.2
|
|
7 a
|
20040342
|
Anhui
|
696.6㊣9.5
|
297.2㊣3.5
|
834.6㊣2.5
|
159.5㊣3.5
|
|
8 a
|
40237
|
Shanghai
|
621.5㊣39.2
|
249.6㊣6.6
|
777.4㊣24.6
|
183.6㊣9.7
|
|
9 a
|
4120369
|
Beijing
|
483.3㊣9.4
|
285.3㊣9.5
|
222.2㊣3.3
|
141.3㊣6.4
|
|
10 a
|
20040561
|
Yunnan
|
666.1㊣9.3
|
309.7㊣6.2
|
744.4㊣3.5
|
207.5㊣6.7
|
|
11 a
|
40614
|
Sichuang
|
609.4㊣3.5
|
354.3㊣9.3
|
507.3㊣9.7
|
135.7㊣3.2
|
|
12 a
|
20040307
|
Hebei
|
447.2㊣12.5
|
240.7㊣3.4
|
453.5㊣3.2
|
111.2㊣3.7
|
|
13 a
|
40110
|
Fujiang
|
429.3㊣33.5
|
141.8㊣6.5
|
279.3㊣6.3
|
75.4㊣3.3
|
|
14 a
|
40505
|
Guangxi
|
642.6㊣15.3
|
129.3㊣6.3
|
354.4㊣6.1
|
78.6㊣2.8
|
|
15 a
|
20301
|
Jiangxi
|
507.2㊣6.4
|
237.5㊣3.2
|
369.6㊣3,5
|
81.8㊣3.1
|
|
16 a
|
31101
|
Guangdong
|
675.5㊣2.6
|
144.3㊣6.3
|
300.3㊣6.3
|
87.3㊣6.2
|
|
17 a
|
40343
|
Guangdong
|
576.2㊣15.3
|
141.6㊣6.6
|
72.1㊣3.0
|
ND
|
|
18 a
|
40208
|
Guangdong
|
777.4㊣24.5
|
264.8㊣6.2
|
426.3㊣6.3
|
135.5㊣3.6
|
|
19 a
|
301900
|
Guangdong
|
615.2㊣3.3
|
408.3㊣9.7
|
1863.5㊣27.6
|
399.7㊣6.4
|
|
20 a
|
40427
|
Beijing
|
528.4㊣27.5
|
252.2㊣9.4
|
510.3㊣9.7
|
114.5㊣6.2
|
|
21b
|
200406
|
Shandong
|
2.01㊣0.09
|
1.82㊣0.03
|
2.63㊣0.03
|
1.37㊣0.01
|
|
22 b
|
200407
|
Sichuang
|
5.03㊣0.27
|
1.22㊣0.02
|
4.08㊣0.05
|
3.72㊣0.04
|
|
23 b
|
200308
|
Shanxi
|
7.45㊣0.32
|
2.01㊣0.03
|
5.49㊣0.05
|
3.51㊣0.03
|
|
24 b
|
200309
|
Liaoning
|
0.44㊣0.02
|
0.17㊣0.01
|
0.23㊣0.00
|
0.07㊣0.00
|
|
25 b
|
200409
|
Henan
|
1.79㊣0.08
|
1.16㊣0.02
|
0.98㊣0.01
|
0.48㊣0.01
|
|
26 b
|
200408
|
Zhejiang
|
0.40㊣0.02
|
0.36㊣0.01
|
0.17㊣0.00
|
0.10㊣0.00
|
|
27 b
|
200107
|
Shanxi
|
2.23㊣0.11
|
2.03㊣0.04
|
1.81㊣0.02
|
3.95㊣0.04
|
|
28 b
|
200309
|
Liaoning
|
2.37㊣0.10
|
1.27㊣0.03
|
2.59㊣0.03
|
2.13㊣0.02
|
|
29 b
|
200402
|
Henan
|
2.45㊣0.12
|
1.09㊣0.02
|
1.53㊣0.01
|
1.34㊣0.01
|
|
30 b
|
200408
|
Shanxi
|
2.03㊣0.09
|
1.41㊣0.03
|
0.75㊣0.01
|
0.98㊣0.01
|
|
31 b
|
200407
|
Hebei
|
2.48㊣0.09
|
0.98㊣0.01
|
1.29㊣0.01
|
0.82㊣0.01
|
|
32 b
|
200403
|
Shanxi
|
2.27㊣0.08
|
1.20㊣0.01
|
1.39㊣0.01
|
0.94㊣0.01
|
|
33 b
|
200301
|
Shanxi
|
2.60㊣0.09
|
1.95㊣0.03
|
1.81㊣0.02
|
2.05㊣0.02
|
|
34 b
|
200303
|
Shandong
|
5.11㊣0.14
|
3.29㊣0.05
|
3.09㊣0.04
|
2.41㊣0.02
|
|
35 c
|
040710
|
Shanghai
|
ND
|
ND
|
ND
|
ND
|
|
36d
|
040828
|
Guangdong
|
ND
|
ND
|
ND
|
ND
|
|
37 e
|
20031001
|
Tianjing
|
ND
|
2.8㊣0.3
|
5.4㊣0.4
|
ND
|
|
38 e
|
20030618
|
Tianjing
|
ND
|
2.9㊣0.2
|
5.5㊣0.5
|
ND
|
|
39 e
|
20040218
|
Tianjing
|
ND
|
2.7㊣0.2
|
5.4㊣0.4
|
5.3㊣0.4
|
|
40 e
|
20040508
|
Ttianjing
|
2.7㊣0.2
|
2.7㊣0.3
|
5.3㊣0.3
|
5.2㊣0.3
|
a: Fufang Danshen tablet, b: Danshen crude
drug, c: Danshen injection,
d: Xiangdan injection, e: Compound Danshen dripping
pill, f Content = mean㊣SD(n=3), units of Danshen crude drug is mg/g,
units of FDT is µg(tanshinone)/300mg tablet, units of CDDP is
µg(tanshinone)/27mg dripping pill, units of DSI and XDI is mg/10ml injection, gND:
not detected, h: Batch Number used in the TCMPs and Harvest time
used in Danshen crude drug.
ACKNOWLEDGEMENTS
We thank the Ministry of Science and Technology of China
(2002BA906A, 2002DEA 20021 and
2001BAC01A56) and National
Administration of Traditional Chinese Medicine (2004ZX01) for financial support.
﹛
REFERENCES
[1]
National
Commission of Chinese Pharmacopoeia, Pharmacopoeia of Peoples Republic of China, part I, The
Chemical Industry Publishing House, Beijing,
China, pp 527-528,
2005.
[2] Wasser,
S.; Ho, J.M.S.; Hui, K. and Tan, C.E.L., Salvia miltiorrhiza reduces experimentally-induced hepatic
fibrosis in rats. J. Hepatol, 29(5): 760-771, 1998.
[3] Liu,
J.; Shen, H.M. and Ong, C.N., Salvia
miltiorrhiza inhibits cell growth and induces apoptosis in human hepatoma
HepG(2) cells. Cancer Lett, 153(1-2):
85-93, 2000.
[4] Ji,
X.Y.; Tan, B.K.H.; Zhu, Y.C.; Lin, W. and Zhu, Y.Z., Comparison of
cardioprotective effects using ramipril and Danshen for the treatment of acute
myocardial infarction in rats. Life Sci,
73(11): 1413-1426, 2003.
[5] Zhang,
J.L.; Cui, M.; He, Y.; Yu, H. L. and Guo, D.A., Chemical fingerprint and
metabolic fingerprint analysis of Danshen injection by HPLC每UV and HPLC每MS
methods. J. Pharm. Biomed. Anal, 36(5): 1029-1035, 2005.
[6] Pei,
W.J., Zhao, X.F., Zhu, Z.M., Lin, C.Z., Zhao, W.M. and Zheng, X.H.; Study of
the determination and pharmacokinetics of Compound Danshen Dripping Pills in
human serum by column switching liquid chromatography electrospray ion trap
mass spectrometry. J. Chromatogr. B Analyt Technol Biomed Life Sci, 809(2): 237每242,
2004.
[7] Liu,
G.Q., Wang, L., Ru, Z.Y. and Yang, H.D., Effect of salvia miltiorrhiza on angiogenesis in dogs with experimental
acute myocardial infarction. Chi.
J Hosp. Pharm, 19(11): 653-655, 1999.
[8] Luo,
X.J., Bi, K.S., Zhou, S.Y. and Zhang, S.H., A Brief Account of the Study on Fufangdashen Tablets. Chin. Tradi. Patent Med, 23(5): 371-373,
2001.
[9] Huang,
H., Tang, Y. and Zhang, A.,C.; The
Study of Committed Differentiation from Adult rMSCs into Neuron-like Cells Induced by Xiangdan Injection in
Vitro. J. Chin. Med. Mater, 8:
585-589, 2004.
[10] Ng
T.B., Liu F. and Wang Z.T.; Antioxidative Activity of Natural Products from
Plants. Life Sci, 66(8): 709-723,
2000.
[11] Kang,
B.Y., Chung, S.W., Kim, S.H., and Ru S.Y., The effect of a ceramide analog, N-acetylsphingosine
on the induction of proliferation and IL-2 synthesis in T cells from young and
old F344 rats. Immunopharmacology,
49(3): 355每361, 2000.
[12] Wang,
H., Gao, X.M. and Zhang, B.L.; Tanshinone: An inhibitor of proliferation of
vascular smooth muscle cells. J Ethnopharmacol, 99(1): 93-98, 2005.
[13] Du,
J.R., Li, X., Zhang, R. and Qian, Z.M.; Tanshinone inhibits intimal hyperplasia
in the ligated carotid artery in mice. J Ethnopharmacol, 98(3): 319-322,
2005.
[14] Sun, J., Tan,
B.K. and Huang, S.H., Whiteman M, Zhu YZ; Effects of natural
products on ischemic heart diseases and cardiovascular system. Acta Pharmacol
Sin, 23(12): 1142 - 1151, 2002.
[15] Cao,
E.H., Liu X.Q., Wang J.J. and Xu N.F., Effect of natural antioxidant tanshinone
II-A on DNA damage by lipid peroxidation in liver cells. Free Radic Biol Med, 20(6): 801-803, 1996.
[16] Xu, M., Wang,
Y.P., Luo, W.B.and Xuan L.J.; Salvianolate inhibits proliferation
and endothelin release in cultured rat mesangial cells. Acta Pharmacol Sin, 22(7): 629-33, 2001.
[17] Chen,
B., Zhu, M., Xing, W.X., Liu, L.L. and WU, Y.T.; SFE-CGC Determination of Tanshinone in Salvia miltiorrhiza Bunge. Acta
Pharm Sin, 36(1): 55 每 57, 2001.
[18] Zhen,
L.Y., Chen, M. and Deng, Q.H., Study on
the chromatography fingerprint of compound Tanshen tablet for its quality
assessment. Acad J
Guangdong College of Pharm, 18(3): 182-184, 2002.
[19] Liu,
S.X., Jia, Y.Y. and Yu, J.H., Determ
ination of Protocatechuic Aldehyde in Guanshu Granules by TLC-scanning. Shizen J Tradit Chin Med Res, 8(2): 140-141ㄛ1997.
[20] Wu,
S.X. and Huang, X.L.; Determina tion of
Tanshinone IIA in compound Tanshen tablet by TLC-scanning Shizen J Tradit
Chin Med Res, 8(5): 415-416, 1997.
[21] Yu,
T. and Wu, X.Q., Determination of
Tanshinone IIA in compound Tanshen granule by HPLC. J Chin Mater Med, 21(8): 421-422, 1998.
[22] Shi,
Z.H., He, J.T. and Chang, W.B.; Micelle-mediated extraction of tanshinones from
Salvia miltiorrhiza bunge
with analysis by high-performance liquid chromatography. Talanta, 64(7): 401每407, 2004.
[23] Li,
H.B. and Chen, F., Preparative isolation and purification of six diterpenoids
from the Chinese medicinal plant Salvia
miltiorrhiza by high-speed counter-current chromatography. J Chromatogr A, 925(1-2): 109-114, 2001.
[24] Pan,
X., Niu, G. and Liu, H., Microwave-assisted extraction of tanshinones from Salvia miltiorrhiza bunge with
analysis by high-performance liquid chromatography. J Chromatogr A, 922(1-2): 371-379, 2001.
[25] Qiu,
Z.Y.; Determination of Tanshinone IIA in Changshangfangban
Ointment by HPLC. Chin Tradi Patent Med,
23(1):18-20, 2001.
[26] Watson,
D.G., Control of the quality of analytical methods. In: Watson DG (ed) Pharmaceutical Analysis. Churchill
Livingstone, New York,
pp 11-12. 1999.
﹛
JPPS Contents
Published by the Canadian Society for
Pharmaceutical Sciences.
Copyright © 1998 by the Canadian
Society for Pharmaceutical Sciences.
http://www.cspscanada.org/
CSPS Home |
JPPS Home |
Search |
Subscribe to JPPS
﹛




