J Pharm Pharmaceut Sci
(www.cspscanada.org) 8(3):536-543, 2005
Distribution of Photosensitizers in Bladder
Cancer Spheroids: Implications for Intravesical Instillation of
Photosensitizers for Photodynamic Therapy of Bladder Cancer.
Zhengwen xiao1, christian b. hansen2, 3,
Theresa m. Allen2,
Gerald G. Miller4 and ronald b. moore1
Departments of
1Surgery,
1Oncology, and
2Pharmacology, University
of Alberta, Edmonton, Alberta, Canada
3AltaRex Corporation, Edmonton,
Alberta, Canada.
4Noujaim
Institute for Pharmaceutical Oncology Research,
Faculty of Pharmacy &
Pharmaceutical Sciences, University of Alberta, Edmonton, Alberta, Canada
Received March 1, 2005; Revised September 7,
2005; Accepted September 19, 2005; Published September
28
2005
PDF
Version
ABSTRACT
PURPOSE: uniform intratumor distribution of sufficient photosensitizer is one
of the important aspects of photodynamic therapy for solid tumors.
METHODS: Multicellular spheroids
derived from a human transitional cell carcinoma cell line (MGHU3) were used as
a surrogate system of tiny solid tumors to study intratumor distribution of
photosensitizers. Photosensitizers included Photofrin, hypocrellins
(HBEA-R1/R2, HBBA-R2), aluminum phthalocyanine chloride (AlPC), benzoporphyrin
derivative monoacid ring A (BPD-MA), protoporphyrin-IX (PpIX), and liposomal
formulations of HBBA-R2 and BPD-MA. Spheroids were incubated with various doses
of the above drugs for 1–4 hours, and were examined by confocal microscopy.
RESULTS: Histology showed all cells
were healthy in spheroids less than 400 µm in diameter. Scanning electron
microscopy showed tight cell-to-cell interdigitation in spheroids. HBEA-R1/R2
distributed more uniformly in spheroids than other drugs. Free hypocrellins and
BPD-MA penetrated spheroids centripetally deeper than AlPC, Photofrin, and PpIX.
Liposomal HBBA-R2 and BPD-MA penetrated less than their free formulations.
CONCLUSIONS: The spheroids mimic solid
tumors prior to neovascularization. Based on drug distribution in spheroids,
hypocrellins and BPD-MA appear superior to Photofrin, AlPC and PpIX for
intravesical administration for bladder cancer phototherapy.
Introduction
In photodynamic therapy (PDT) of cancers, a
photosensitizer, light, and oxygen are photochemically interactive
to cause cell death ([1]).
Several mechanisms have been proposed for PDT mediated tumor destruction,
including direct and indirect cell killing ([2],[3]).
Direct cell killing depends on selective accumulation of sufficient amounts of
a photosensitizer in tumor (3). The uptake, distribution and retention
of a sensitizer in tumor are dependent on the route and mode of delivery, as
well as the physicochemical properties (e.g.
lipophilicity) of the drug. For instance, lipophilic photosensitizers need to
be incorporated into delivery vehicles for in
vivo administration, and they are taken up by neoplastic cells partially via a receptor-mediated pathway, and
binding mainly with cellular membranes ([4],[5]).
In situ photoactivation of these
drugs may therefore result in direct cell killing ([6],[7]).
In contrast, hydrophilic sensitizers, such as tri- and tetrasulfonated
porphyrins and phthalocyanines, can be administered as free drugs. They bind in
a noncovalent fashion to plasma proteins (albumin and globulins) and
subsequently localize in vascular stroma of normal and tumor tissues ([8],[9]). Photoactivation of these drugs causes
damage to the microvasculature, leading to vascular stasis and tumor infarction
(indirect cell killing) ([10]).
Damage to the microvasculature is
a prominent in vivo tumor response to
PDT with Photofrin (porfimer sodium), the most widely used photosensitizer (1, 2). Patients treated with Photofrin-based PDT, however,
exhibit prolonged skin phototoxicity ([11]).
This side effect is attributable to prolonged retention of the photosensitizer
in the skin. In addition, following systemic administration, Photofrin-based
PDT causes bladder shrinkage in patients with bladder
cancers ([12]). These side effects have prompted
the search for new photosensitizers and new routes of drug administration. Phthalocyanines,
benzoporphyrin derivatives, and hypocrellins are second-generation photosensitizers
that can be activated by longer wavelengths (> 630 nm) than Photofrin. Furthermore,
the monomeric properties of these second-generation photosensitizers promote
more rapid clearance from normal tissues; therefore prolonged skin phototoxicity may not be problematic ([13],[14],[15]).
Topical administration of
liposomal formulations of photosensitizers not only broadens the application of
potent monomeric, lipophilic photosensitizers, but also facilitates uptake by
tumor cells due to direct contact of the liposomal drug with tumor, thereby
reducing distribution to the reticuloendothelial system (5,[16]). To a great extent, the
efficacy of PDT for bladder cancer depends on the degree of intratumor uptake
of photosensitizers and their phototoxicity to cancer cells. Selection of
appropriate photosensitizing drugs for whole bladder PDT therefore requires
knowledge of dose- and time-dependent accumulation of the drugs in tumor, as
well as their phototoxicity. Multicellular spheroids have been used as
surrogates of tiny tumors for studying distribution and efficacy of chemo- and
radio-therapeutic agents ([17],[18],[19]). In this study, the distribution and
retention of several second-generation photosensitizers, as well as their
liposomal formulations, in human bladder cancer cell spheroids were
investigated. The results were compared with those of Photofrin, to explore the
potential of these new photosensitizers for PDT of superficial bladder cancers
following intravesical administration.
Materials and Methods
tumor cells and spheroid growth kinetics. The spheroids were cultured from a
moderately differentiated human transitional cell carcinoma cell line (MGHU3).
MGHU3 cells were generously provided by Dr. Y. Fradet at the University of
Laval, Quebec. Spheroids were generated by adding 2 × 106 cells to
60 mL of Dulbecco’s modified Eagle’s medium [D-MEM (Gibco/BRL, Burlington,
ON)], supplemented with 10% fetal calf serum and antibiotics. The spheroids
grew in spinner flasks on a stir-plate under standard cell culture conditions
(37°C, 5% CO2). Half of the medium was replaced with fresh medium 4
days later and every other day thereafter. To establish the growth kinetics of
the spheroids, samples were taken every two days. The spheroids’ size was
measured by microscopy. Spheroids reaching 300 µm in diameter (at 8 – 10 days)
were processed for histology to determine if there were necrotic cells in the
center, or for scanning electron microscopy (SEM) to study cell-cell
connections on the spheroid surface and in its cross section.
Photosensitizers. (1) Photofrin was provided by QLT Inc. (Vancouver, BC).
It was dissolved in 5% dextrose (Abbott laboratories,
Montreal, QC),
then diluted with serum-free medium immediately before incubation with
spheroids. (2) Hypocrellin B (HB, Altarex Corp. Edmonton, AB) included HBEA-R1/R2
(ethanolaminated HB, Mr
614), HBBA-R2 (n-butylaminated HB, Mr
636), and liposomal HBBA-R2. We have previously reported the physical and chemical
properties of hypocrellins in detail (15). Free hypocrellins were first dissolved in ethanol,
and then diluted with serum-free medium. The
liposomal preparations will be discussed below. (3) Benzoporphyrin
derivative monoacid ring A (BPD-MA, Mr
718) and liposomal BPD-MA were provided by QLT Inc. BPD-MA was dissolved in
dimethyl-sulfoxide (DMSO) and then diluted with serum-free medium prior to use. Liposomal BPD-MA (2.0 mg/ml stock solution) was further diluted with serum-free medium immediately
before use. (4) aluminum
phthalocyanine chloride (AlPC) was purchased from Acros Organics (Mr 575), and (5)
protoporphyrin IX (PpIX, Mr
562.7) was purchased from Sigma Chemical Co. (St. Louis, MO).
AlPC and PpIX were first dissolved in DMSO and then diluted with serum-free
medium.
Liposomal
hypocrellin formulation. The procedures of liposome preparation were
described previously ([20]). Dipalmitoyphosphatidylcholine (DPPC) was
purchased from Avanti Polar Lipids, and
maleimide-PEG2000-distearoylphosphatidylethanolamine (DSPE) was purchased from
Shearwater Polymers Inc. (Huntsville,
AL). Long circulating, sterically
stabilized liposome (SL) formulation of HBBA-R2 was composed of
DPPC/maleimide-PEG2000-DSPE (94:6 molar ratio). HBBA-R2 was loaded into
pre-formed SL at a 15:1 lipid to drug molar ratio. Liposomes were prepared by
hydrating a dried thin film of lipids to a final concentration of 10 mM lipid with 20 mM HEPES 140 mM NaCl, pH
7.4. HBBA-R2 dissolved in solvent (20 mM
in methanol) was heated to 65°C and injected dropwise, into a 65°C solution of liposomes. The resulting
hypocrellin-liposomes were extruded through 100 nm and 80 nm polycarbonate
membranes using a Lipex® Biomembranes extruder (Vancouver, BC)
to give an average vesicle size of 100 nm. The liposomes were then purified by
gel filtration and assayed for lipid and drug concentrations. A final lipid to
drug molar ratio of 20:1 was typically obtained. Liposomal HBBA-R2 was further
diluted with serum-free medium before use.
photosensitizer distribution in spheroids. Spheroids 200- to 400-µm in diameter were
incubated with Photofrin up to 15 µg/ml or graded doses (0 – 20 µM) of other photosensitizers and
liposomal drugs for different time-points (1 – 4 h). Plain liposomes (~300 µM lipids) were also incubated with
spheroids for lipid-only controls. More than 5 spheroids were incubated in a
35-mm suspension culture dish (Corning,
NY) in 2 ml of solution
containing various drugs at 37°C. At the end of the incubation, the spheroids
were gently washed with phosphate-buffered saline (PBS) three times, and imaged
using a confocal laser-scanning microscope (CLSM). The CLSM system (Molecular
Dynamics, CA) consisted of an argon-krypton laser, a Nikon inverted microscope,
and ImageSpaceŇ software (version 3.2, Molecular Dynamics, CA) to
analyze the images (analyzing intensity profile, 3–dimension reconstruction). Confocal parameters are listed as
follows: 20´/0.55 objective lens; 488/568 nm exciting wavelengths
(for AlPC, 647 nm was used); 488/568 nm beam-splitter (for AlPC, 647 nm
beam-splitter was used); 590 nm long-pass (LP590) barrier filter for
fluorescence detection (for AlPC, a LP660 filter was used). The interval
between optical slices was 2 – 3 µm. Five typical spheroids from each
time-point and drug concentration were scanned from the surface (top) to the
center, and the images were stored for further analysis.
Fluorescence intensity histograms
in the central sections of each spheroid were created using ImageSpaceŇ software. From these histograms, areas under
the intensity vs. spheroid diameter
curve (AUC) were calculated with the trapezoid area formula ([21]), and normalized by each spheroid’s
diameter. These averaged AUCs represent drug accumulation in spheroids.
Results
Growth characteristics of MGHU3 spheroids
the spheroids’ growth kinetics is shown in
Figure 1.
At 8 – 10 days, the average
diameter of the spheroids was around 300 µm. The spheroids continue to grow to
600 – 700 µm at 4 weeks, and attach to each other thereafter (Figure 1). Histological
examination showed all the cells from the periphery to the center were healthy
in spheroids less than 400 µm in diameter.
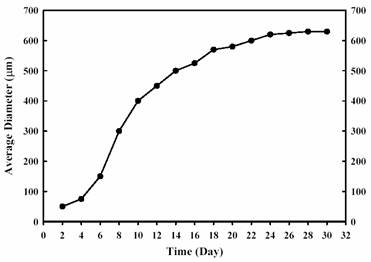
Figure
1. Growth kinetics of MGHU3
spheroids cultured in spinner flasks.
In those spheroids greater than 500 µm, a
central zone of degenerative changes (hypoxia or necrosis) was observed (data
not shown). Scanning electron microscopy showed a network-like extracellular
matrix covering the spheroid, and tight cell-cell interdigitation of microvilli
in the cross section (Figs 2, 3).
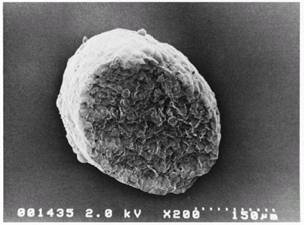
Figure
2. SEM microphotograph of a
MGHU3 spheroid showing an extracellular matrix network covering the surface,
which models a small tumor prior to neovascularization.

Figure
3. SEM microphotograph of a
cross section of a spheroid-displaying tumor cells in tight contact with
interdigitation of microvilli.
Intraspheroid
distribution of photosensitizers
Figure 4 shows a series of confocal sections
scanned from the top surface to the center of a spheroid incubated with
HBEA-R1/R2. HBEA-R1/R2 was distributed from the surface to the center of a
spheroid with fluorescence intensity in the peripheral sections slightly higher
than in the central sections.
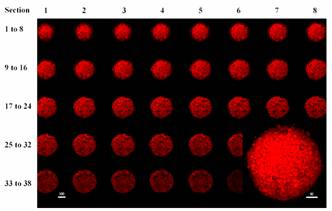
Figure
4. Serial confocal sections
from the top surface to the center of a MGHU3 spheroid incubated with 10 mM HBEA-R1 for 2 hours. Inset, a 3-dimension
projection of the spheroid showing the spheroid surface. (Bars denote mms).
Penetration and distribution of other
photosensitizers in the central sections of spheroids are displayed in Figures
5A–H, and a representative fluorescence intensity histogram across a central
section is shown in Figure 5C.
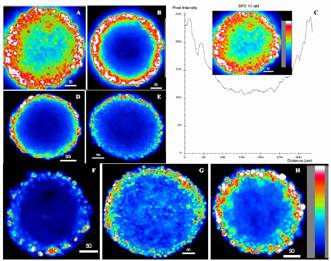
Figure
5. Pseudo-color confocal
images showing penetration, distribution and intensity profile in the central
sections of spheroids incubated with photosensitizers at 37şC for 4 hours. (A),
BPD-MA 10 mM (7.18 mg/ml);
(B), liposomal BPD-MA 10 mM; (C), histogram profile of A; (D), AlPC 10 mM (5.75 mg/ml); (E),
Photofrin 15 mg/ml; (F), PpIX 10 mM (5.6 mg/ml); (G), HBBA-R2 10 mM (6.36 mg/ml); (H),
SL-HBBA-R2 10 mM. (Bars denote mms).
Generally, in these color-coded confocal
micrographs, the highest intensity (white) was observed at the spheroid
periphery ranging from one to ten cells in depth, and spheroid centers showed
the lowest intensity (black-purple). Interestingly, BPD-MA (Fig. 5A) and
HBBA-R2 (Fig. 5G) penetrated deeper than AlPC (Fig. 5D), Photofrin (Fig. 5E)
and PpIX (Fig. 5F), so that the intensity level of BPD at the center of a
spheroid reached almost half of that at the periphery (Fig. 5C). For the latter
three drugs, fluorescence was detected only at the spheroid rim (< 3
cellular layers), while at the center virtually no fluorescence was observed
(Fig. 5D, 5E, and 5F). For comparison, spheroids incubated with liposomal
BPD-MA (Fig. 5B) and liposomal HBBA-R2 (Fig. 5H) also showed high levels of
fluorescence at the periphery. However, the intensity levels at the center were
lower than that of their free drugs (Fig. 5B vs. 5A, and Fig. 5H vs.
5G).
To further analyze the
fluorescence distribution quantitatively, the average normalized AUC’s of various
drugs are summarized in Table 1. These AUC’s are indications of drug
accumulation in spheroids for a given drug to examine the time and dose effects.
These AUCs data clearly suggested that spheroids efficiently took up and
accumulated BPD-MA, liposomal BPD-MA, hypocrellins and liposomal HBBA-R2.
The drug accumulation was both time- and dose-dependent,
except HBEA-R1/R2, for which the AUC’s at 2 h already reached the plateau state.
Based on a molar concentration (10 µM
incubated for 4 h), the AUC of BPD-MA was close to that of liposomal BPD-MA;
two times of that of HBBA-R2 and liposomal HBBA-R2; more than four times of
that of AlPC; ten times of that of Photofrin (15 µg/ml) and 18 times of that of
PpIX.
Discussion
Whole bladder PDT has been proven as an
effective treatment modality for refractory superficial bladder cancer (12). In whole bladder PDT, sufficient accumulation of
the photosensitizer in tumor tissue, compared to underlying normal tissues, is
very important to ablate the tumor while maintaining normal bladder function. The
main objective of intravesical instillation of photosensitizers is to increase
the local tissue (i.e. tumor)
concentration and decrease systemic uptake of the drug, and thus reduce the
undesired side effects (bladder contracture and prolonged photosensitivity). As
there is limited data available on the distribution of photosensitizers in
tumors after intravesical administration, we carried out the study in order to
determine the distribution pattern of both first and second-generation
photosensitizers in MGHU3 spheroids. These spheroids resemble small residual
bladder tumors prior to vascularization. This study provides a first step for
screening photosensitizers with potential intravesical application.
Many factors can effect
photosensitizer distribution in cells and tumor. For photosensitizer per se,
the structure determines its biological parameters, such as lipophilicity.
According to structures, Boyle and Dolphin classified photosensitizers into
three major groups ([22]): (1) Hydrophobic sensitizers are
defined as those bearing no charged substituents and which have negligible
solubility in water or alcohol. (2) Hydrophilic sensitizers have three or more
charged substituents and are freely soluble in water at physiological pH. (3)
Amphiphilic sensitizers have two or less charged substituents and are soluble
in alcohol or water at physiological pH. Therefore, AlPC falls into the
hydrophobic group. PpIX, BPD-MA, and HBBA-R2 are on the borderline between the
hydrophobic and amphiphilic groups. HBEA-R1 is amphiphilic. Photofrin is on the
borderline between amphiphilic and hydrophilic. The photosensitizers tested in
this report included those promising second-generation drugs, as well as the
first-generation sensitizer, Photofrin. Hypocrellin B derivatives (HBEA-R1,
HBBA-R2) have been documented as potent photosensitizers in vitro (15) and in vivo ([23],
[24]). However, the more lipophilic HBBA-R2,
like BPD-MA (14) and AlPC (13), is not suitable for in vivo administration without being incorporated into liposomes or other suitable carriers (5). Photofrin is a mixture of oligo-porphyrins.
PpIX was selected because it is an endogenous sensitizer derived from
5-aminolevulinic acid, which has been used in pre-clinical and clinical trials
([25], [26],
[27]). In general, a
concentration-dependent, diffusion driven penetration and accumulation have
been demonstrated for each compound tested (Table 1, Fig. 5). In confocal
microscopy, the fluorescence levels at spheroid rim are always higher than that
in spheroid center (Fig. 5). HBEA-R1 penetrates deeper and distributes more
uniformly into spheroids than its analog, HBBA-R2 (Fig. 4), probably because
the latter is more lipophilic. HBBA-R2 may have higher affinity for membranous
structures, which retards its penetration into the spheroid center. Similar
result of decreased penetration of hypericin with increased lipophilicity has
been reported ([28]).
Furthermore, the network of hydrophilic extracellular matrix on the spheroid
surface may also impede the penetration of lipophilic
compounds ([29]). This extracellular matrix network
consists of proteoglycans and glycosaminoglycans, and is not expressed in
monolayer cells (28, 29). Photofrin can only penetrate about three cell
layers, which may be attributed to its large oligo-porphyrin structures ([30]). Although BPD-MA has larger molecular
size than AlPC and PpIX, it penetrates better than the later two (Fig. 5). Thus,
molecular size and lipophilicity are just two of the many factors affecting
penetration. Other factors also include drug concentration and availability
(aggregation), incubation time, cell cycle, spheroid (or tumor) structures, and
drug carriers used. One could speculate that each drug might have a unique
distribution pattern, particularly in the diverse clinical settings.
The liposomal formulations of
BPD-MA and HBBA-R2 used in this study do not have a drug-targeting role, and
provide merely carriers whereby BPD-MA and HBBA-R2 can be administered in
monomeric forms. Aggregation can reduce a photosensitizer's bioavailability in vivo, and undermine its capacity to
interact with light and therefore its effectiveness ([31]).
Compared to free drug, both liposomal BPD-MA and HBBA-R2 have poor penetration
into the spheroids, whereas the fluorescence accumulation at the spheroid rim
is high (Fig. 5). However, the normalized drug accumulation is similar for both
free and liposomal formulations (Table 1). This could be explained in that
liposomes may transiently keep the photosensitizers from penetrating due to
their size, and the photosensitizers may be taken up as liposome-drug packages
by cells in the spheroid rim. Apart from as carriers, liposomes conjugated with
monoclonal antibody directed against tumor cells might be exploited for
site-specific immunophotodynamic therapy of bladder cancer ([32]).
Multicellular spheroid provides a
good 3-dimensional model for drug distribution studies. Ideally, spheroids
should be used to test phototoxicity in an environment mimicking small tumors.
However, in our practice, we found that it was difficult to accurately assess
the results by using spheroid for phototoxicity study, especially for comparing
different kinds of photosensitizers. These limitations include: (a) when
spheroids reach sizes of 400 mm or greater, the cells in the center are
resistant to photodynamic therapy (PDT) due to hypoxia ([33]).
Phototoxicity is a reciprocal effect of light, drug and oxygen. Since there is
no vasculature inside the spheroids to provide tissue oxygen, PDT can easily
deplete oxygen and have virtually no effect on cells in the spheroid center (33). The proportion of hypoxic cells is dependent on the
spheroid size. The larger the spheroid, the more hypoxic cells versus
oxygenated cells. Therefore, to compare phototoxicity among different
photosensitizers, spheroid sizes selected should be the same. Practically, it
is very difficult, if not impossible. (b) The cells in the spheroid rim
accumulated much higher levels of photosensitizers than the cells in the center
did (demonstrated in this study). Trypsinization of the spheroids after PDT
destroys the 3-D configuration of the spheroids, and mixes up the cells from
the rim with the cells from the center. Subsequently sampling of the cells for
clonogenic experiments may either overestimate the phototoxicity (by sampling
more cells from the rim) or underestimate the phototoxicity of the drug (by
sampling more cells from the center of the spheroid). Using monolayer cells to
preliminarily screen potent photosensitizers has some advantages, because the
three major factors in PDT (drug dose, light dose and oxygen) can be strictly
controlled. In vivo studies comparing
tissue distribution of liposomal hypocrellin vs. hypocrellin dissolved in DMSO/saline demonstrated that
liposomal hypocrellin reached higher drug levels in tumor, but took a longer
time to reach the maximal level than the free drug ([34]).
Similarly, the present study also shows comparable drug accumulation in
spheroids between liposomal and free BPD-MA and HBBA-R2 if the incubation time
is longer than 2 hours.
In conclusion, the multicellular
MGHU3 spheroids resemble small tumors prior to neovascularization, so that drug
distribution in spheroids may mimic the situation in residual bladder tumor.
Based on drug distribution in the spheroids in
vitro, BPD-MA and hypocrellin B derivatives seem to be the most promising
candidates for intravesical administration for PDT of bladder cancer. Liposomes
can be used as carriers to deliver these potent lipophilic photosensitizers in vivo. The liposomal formulations of
HBBA-R2 and BPD-MA may be utilized for immunophotodynamic therapy of bladder
cancer, given an appropriate targeting antibody.
Acknowledgments
Funding support from the Alberta Cancer Board,
the Edmonton Civic Employees’ Charitable Fund, and the Alberta Heritage
Foundation for Medical Research is gratefully acknowledged. Special thanks are
to Mr. Bhatnagar for the excellent assistance with confocal microscopy.
References
-
Henderson B W and Dougtherty T
J. How does photodynamic therapy
work? Photochem. Photobiol.,
55: 145-157, 1992.
-
Doughery T J, Gomer C J,
Henderson B W, et al. Photodynamic
therapy. J Natl. Cancer Inst.,
90: 889-905, 1998.
-
Van Hillegersberg R, Will JK and
Wilson JHP. Current status of
photodynamic therapy in oncology
(review). Drugs. 48
(4): 510-527, 1994.
-
Love WG, Duk S, Biolo R, Jori G
and Taylor PW. Liposome-mediated
delivery of photosensitizers:
localization of Zinc
(II)-Phthalocyanine within implanted
tumors after intravenous
administration. Photochem.
Photobiol., 63: 656-661,
1996.
-
Reddi
e.
role of delivery
vehicle for photosensitizers in the
photodynamic therapy of tumors
(review). J. Photochem. Photobiol.
B: Biol. 37: 189-195, 1997.
-
[Peng Q, Moan J, Farrants G,
Danielsen HE and Rimington C.
Localization of potent
photosensitizers in human tumor LOX by
means of laser scanning microscopy.
Cancer Lett., 53:
129-139, 1990.
-
Cuomo V, Jori G, Rihter B,
Kenney ME and Rodgers MAJ.
Liposome-delivered
Si(IV)-naphthalocyanine as a
photodynamic sensitizer for
experimental tumors: pharmacokinetic
and physiotherapeutic studies. Br.
J. Cancer. 62: 966-970,
1990.
-
Kessel D, Thompson P, Saatio K
and Nantwi KD. Tumor localization and
photosensitization by sulfonated
derivatives of tetraphenyporphine.
Photochem. Photobiol., 45:
787-790, 1987.
-
Jori G and Reddi E. Strategies
for tumor targeting by photodynamic
sensitizers. In Photodynamic
Therapy of Neoplastic Disease
(Edited by D. Kessel), pp. 117-130.
CRC Press, Boca Raton, FL. 1990.
-
Henderson BW, Waldow SM, Mang
TS, Potter WR, Malone PB and Dougherty
TJ. Tumor destruction and kinetics of
tumor cell death in two experimental
mouse tumors following photodynamic
therapy. Cancer Res., 45:
572-576, 1985.
-
Dougherty TJ, Cooper MT and Mang
TS. Cutaneous phototoxic occurrences
in patients receiving Photofrin.
Lasers Surg. Med., 10:
485-488, 1990.
-
Nseyo UO, Shumaker B, Klein EA
and Sutherland K. for the bladder
Photofrin study group. Photodynamic
therapy using porfimer sodium as an
alternative to cystectomy in patients
with refractory transitional cell
carcinoma in situ of the bladder.
J. Urol. 160, 39-44, 1998.
-
Ben-Hut E and Rosenthal I. The
phthalocyanines: a new class of
mammalian cell photosensitizers with a
potential for cancer phototherapy.
Int. J. Radiat. Biol., 47:
145-147, 1995.
-
Sternberg E and Dolphin D. An
overview of second generation drugs
for photodynamic therapy including
BPD-MA. In Spinelli P, Dal Fante M,
Marchesini R, eds. Photodynamic
Therapy and Medical Lasers.
Excerpta Medica 470-474, 1993.
-
Estey EP, Brown K, Diwu Z, Liu
J, Lown JW, Miller GG, Moore RB, Tulip
J and McPhee MS. Hypocrellins as
photosensitizers for photodynamic
therapy: a screening evaluation and
pharmacokinetic study. Cancer
Chemother. Pharmacol., 37:
343-350, 1996.
-
Yarosh DB. Liposomes in
investigative dermatology (review).
Photodermatol. Photoimmunol.
Photomed., 17: 203-212,
2001.
-
Knuchel R, Hofstadter F, Jenkins
WEA and Masters JRW. Sensitivities of
monolayers and spheroids of the human
bladder cancer cell line MGH-U1 to the
drugs used for intravesical
chemotherapy. Cancer Res.,
49: 1397-1401, 1989.
-
Erlichman C and Vidgen D.
Cytotoxicity of adriamycin in MGH-U1
cells grown as monolayer cultures,
spheroids, and xenografts in
immune-deprived mice. Cancer Res.,
44: 5369-5375, 1984.
-
Sasaki T, Yamamoto M, Yamaguchi
T. and Sugiyama S. Development of
multicellular spheroids of Hela cells
cocultured with fibroblasts and their
response to X-irradiation. Cancer
Res., 44: 345, 1984.
-
Allen TM and Hansen CB.
Pharmacokinetics of stealth versus
conventional liposomes: effect of
dose. Biochim. Biophys. Acta.,
1068: 133-141, 1991.
-
Stewart J. Calculus. 2nd
Edition. Brooks/Cole Publishing Co.
Pacific Grove, CA. 1991.
-
Boyle R and Dolphin D. Structure
and biodistribution relationships of
photodynamic sensitizers (review).
Photochem. Photobiol., 64:
469-485, 1996.
-
Diwu Z. Novel therapeutic and
diagnostic applications of
hypocrellins and hypericins.
Photochem. Photobiol., 61:
529-539, 1995.
-
Miller GG, Brown K, Ballangrud
AM, Barajas O, Xiao Z, Tulip J, Lown
JW, Leithoff JM, Allalunis-Turner MJ,
Mehta R.D. and Moore R.B. Preclinical
Assessment of Hypocrellin B and
Hypocrellin B Derivatives as
Sensitizers for Photodynamic Therapy
of Cancer: Progress Update.
Photochem. Photobiol., 65 (4):
714-722, 1997.
-
Xiao Z, Tamimi Y, Brown K, Tulip
J and Moore RB. Interstitial
photodynamic therapy in subcutaneously
implanted urologic tumors in rats
after intravenous administration of
5-aminolevulinic acid. Urol. Oncol.,
7 (3): 125-132, 2002.
-
Waidelich R, Stepp H.,
Baumgartner R, Weninger E, Hofstetter
A and Kriegmair M. Clinical experience
with 5-aminolevulinic acid and
photodynamic therapy for refractory
superficial bladder cancer. J.
Urol., 165: 1904-1907,
2001.
-
Fukuda H,
Casas A and Batlle A. Aminolevulinic
acid: from its unique biological
function to its star role in
photodynamic therapy. Int. J. Biochem.
Cell Biol., 37(2):272-276,
2005.
-
Huygens A, Huyghe D, Bormans G,
Verbruggen A, Kamuhabwa AR, Roskams T.
and de Witte PAM. Accumulation and
photocytotoxicity of hypericin and
analogs in two- and three-dimensional
cultures of transitional cell
carcinoma cells. Photochem. and
Photobiol., 78(6): 607–614,
2003.
-
Glimelius B, Norling B, Nederman
T and Carlsson J. Extracellular
matrices in multicellular spheroids of
human glioma origin: increased
incorporation of proteoglycans and
fiberonectin as compared to monolayer
cultures. Acta Pathol. Microbiol.
Scand. 96: 433-444, 1988.
-
West CML. Size-dependent
resistance of human tumor spheroids to
photodynamic treatment. Br. J.
Cancer 59: 510-514, 1989.
-
Aveline BM, Hasan T and Redmond
RW. The effects of aggregation on the
photophysical properties of
benzoporphyrin derivative-monoacid
ring A (BPD-MA). J. Photochem.
Photobiol. B: Biol., 30:
161-166, 1995.
-
Miller GG and Lown JW.
Immunophotodynamic therapy: current
developments and future prospects
(review). Drug Develop. Res.,
42: 182-197, 1997.
-
Kamuhabwa AA, Huygen A, de Witte
PA. Photodynamic therapy of
transitional cell carcinoma
multicellular tumor spheroids with
hypericin. Int. J. Oncol.
23:1445-1450, 2003.
-
Wang ZJ, He YY, Huang CG, Huang
JS, Huang YC, An JY, Gu Y and Jiang
LJ. Pharmacokinetics, tissue
distribution and photodynamic therapy
efficacy of liposomal-delivered
hypocrellin A, a potential
photosensitizer for tumor therapy.
Photochem. Photobiol., 70:
773-780, 1999.




