J Pharm Pharmaceut Sci (www.cspscanada.org) 8(3):507-515, 2005
Phytopreventative Anti-Hyperlipidemic Effects Of Gynostemma Pentaphyllum In Rats
Samer Megallia,
Fugen Aktanb, Neal M. Daviesc, Basil D. Roufogalisa
aFaculty of Pharmacy and Herbal Medicines Research and
bFaculty of Pharmacy,
cCollege of Pharmacy, Department of Pharmaceutical Sciences,
Received May 7, 2005; Revised September 12, 2005; Accepted September 13, 2005; Published September 16, 2005
Corresponding
Author:
Sam
Megalli, Faculty of Pharmacy,
Pharmacy building A15, The University of Sydney, NSW 2006,
Abstract Purpose: Gynostemma pentaphyllum is widely used in traditional Chinese medicine. Preliminary studies indicate Gynostemma isolated triterpine glycosides lower cholesterol. Our studies examine anti-hyperlipidemic effects of gypenosides. Methods: Gynostemma activity was examined in poloxamer P407 induced hyperlipidemia in rats. Results: 1 g/kg P407 induced plasma triglyceride (25 fold), total cholesterol (6 fold), low density lipoprotein cholesterol (LDL) (7 fold), high density lipoprotein cholesterol (HDL) (1.6 fold), and nitrite (8 fold). After acute (4 days) and chronic (12 days) oral administration the gypenoside extract (250 mg/kg) reduced triglyceride (53% and 85%, respectively) and total cholesterol levels (10% and 44%, respectively). No significant effects on LDL or HDL cholesterol were observed. The gypenosides reduced nitrite ~80%. Similar results were obtained with atorvastatin (75 mg/kg for 4 days); except that LDL cholesterol was reduced (17%) and HDL cholesterol increased. 50% of lipoprotein lipase (LPL) plasma activity was inhibited by ~20 μM P407. Gynostemma had no effect on LL, however, it reversed the P407 inhibition of LPL activity in a concentration-dependent manner, with a 2-fold increase at ~10 μg/ml. Conclusions: These studies demonstrate efficacy of Gynostemma pentaphyllum in lowering triglyceride, cholesterol and nitrite in acute hyperlipidemia. The results suggest further investigations of Gynostemma gypenosides are warranted to examine the mechanisms of this activity.
Introduction
Gynostemma pentaphyllum (Thumb.) Makino (GP) (Jiaogulan-Chinese name)
is an herbaceous vine plant of the cucurbitaceous family and is distributed
naturally in shaded and humid places (1). In traditional Chinese medicine it is
indicated for heat clearing, detoxification, and as an anti-tussive and
expectorant for relieving cough and chronic bronchitis. In Japan it is
indicated as a diuretic, antipyretic, anti-inflammatory and tonic. GP contains
saponins (triterpene glycosides or gypenosides); more than 100 dammarane-type
glycosides, also called gypenosides, have been isolated and identified (2). A
general structure of dammarane-type gypenoside is illustrated in
Figure 1. Some
of these saponins are the same as those from Panax ginseng (ginsensides) (3-4).
Flavonoids such as rutin are also found. The medicinal properties of GP have
been mainly attributed to the saponins (2).
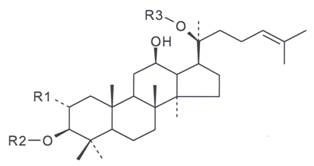
Figure 1: General Structure of Dammarane-Type
Gypenosides. Gypenoside consists of both the hydrophobic sapogenin part and the
hydrophilic sugar part in the molecule (where R1 and R2 = glucose, rhamnose; R3
= glucose, xylose).
Poloxamer 407 (Pluronic RF-127)
has been used to induce hyperlipidemia in rats. P407 is a biocompatible,
non-ionic surfactant (5) is considered non-toxic and safe in animal chronic
administration for long term studies (6). In the rat, once daily intraperitoneal
(ip) injections of 0.33 mg/kg P407 for four consecutive days resulted in the
increase of monocyte numbers with no other toxicological complications (7). In
another study, 0.5 g/kg of P407 was given to C57BL/6 mice every third day for
200 days, without any significant weight loss or alterations in the liver
enzymes aspartate aminotransferase (AST) and alanine aminotransferase (ALT) (8).
The increase in triglycerides (TG) seen following P407 injection is mainly due
to the inhibition of TG degradation, due to a direct inhibitory effect on lipoprotein
lipase (LPL), the enzyme responsible for TG degradation. However, the effect of
P407 on hypercholesterolemia suggests that the elevations of cholesterol could
be mediated through indirect activation on HMG-CoA reductase (6).
Herbal medicines and nutraeuticals are being increasingly accepted and utilised in the Western medicine to treat and/or prevent various diseases. In this present study, we investigate the effect of gypenosides isolated from Gynostemma pentaphylumm on hyperlipidemia and nitrite levels in an acute model of hyperlipidemia. In addition, in vitro and in vivo studies have been utilised to further explore the possible modes of action of Gynostemma pentaphyllum.
Materials and Methods
Purified gypenoside
extract was provided by Ankang
Pharmaceutical Institution of Beijing Medical University, People’s Republic of
China. The Ankang extract in a capsule formulation was dissolved in 90% ethanol
and thoroughly mixed with a magnetic stirrer then filtered twice using filter
paper and evaporated down using a Buchi Rotavapor R-114. The extract was then
dried in a vacuum oven and kept in a bell jar with silica gel. A final purity
of approximately 90% gypenosides was obtained.
Poloxamer 407, also called Pluronic RF-127,
was donated by BSAF Australia. The poloxamer 407 was made at a final
concentration of 30% (w/w) by dissolving the powder in distilled cool water;
the solution was then kept refrigerated overnight to facilitate its
dissolution. Needles and syringes used to administer P407 were cooled prior to
administration to prevent P407 gelation within the syringe. Poloxamer was
administered ip to the rats. Atorvastatin was purchased in a tablet form at
strength 80 mg through Australian Pharmaceutical Industries in Northmead,
Sydney. After crushing the tablets, powder was then dissolved in 1% CMC for oral
dosing via gavage.
For the assay of lipoprotein
lipase (LPL), Glycerol tri [9, 10(n)-3H] oleate ([3H] TO)
was obtained from Amersham Biosciences, Sydney (Code TRA191). Lipoprotein
enzyme from bovine milk and cold glyceryl trioleate, also know as Triolein
(TO), and lecithin stored in chloroform (100 mg/ml) were obtained from
Sigma-Aldrich (Australia). Glycerol and 0.2 M Tris-HCl (pH 8.0) containing 3% bovine
serum albumin (BSA) were also obtained from Sigma-Aldrich (Australia). Ethanol
was obtained from Asia Pacific Specialty Chemicals Ltd. Sydney, Australia. Other
materials including ketamine hydrochloride and carboxymethylcellulose (CMC) were
purchased from Sigma-Aldrich (Australia). Total cholesterol (TC), total
triglyceride, (TG) low density lipoprotein cholesterol (LDL) and high density
lipoprotein cholesterol (HDL) measuring kits were obtained from Sigma-Aldrich
(Australia) and Trace Scientific Ltd.(Australia) and used according to
manufacturers instructions after precipitation techniques and modified
enzymatic procedures to plasma samples.(9-11) All other reagents and chemicals
were of analytical grade.
Animal Grouping and Treatments
Experiments were conducted in accordance with
the Animal Ethics Committee at The University of Sydney guidelines, Approval
number L24/5-2001/2/3369.
Sprague-Dawley (S-D) rats were
purchased from Animal Services at the University of Sydney. The rats obtained
were males, 4-8 weeks old and average weight 250-300 g. Rats were housed 3-4
per cage in a temperature controlled (22 ± 1)˚C room, with a light/dark
cycle of 12 hr. For a week following
their receipt, the animals were allowed free access to a standard rat chow diet
and tap water while they were acclimating to the environment. During the
experimentation all rats had free access to standard rat chow and water at all
times unless otherwise stated in the methods section. Gynostemma
pentaphyllum and atorvastatin samples were well mixed in 1% carboxymethylcellulose.
Treatments were administered to rats using oral gavage via a curved feeding needle
(Harvard Apparatus). At the start of the experiment animals were randomly
distributed so that body weights, initial TG, TC and other parameters were
similar in all the experimental groups.
At the end of each study, animals
were sacrificed and blood collected via cardiac puncture for analysis. Rats
were sacrificed after the induction of anaesthesia using 1 ml of ketamine
injection (1 g /10 ml) and sacrificed using a lethal injection of 0.5 ml
concentrated solution of potassium chloride (70%) solution directly into the
heart.
Effect of Acute and Chronic Gynostemma pentaphyllum
Sprague-Dawley
rats were divided into 3 groups (Figure 2). A control (C) group (12 rats), did
not receive any treatment apart from 1% CMC as an oral gavage, a second group
treated with P407 1 g/kg alone (P407) (12 rats) and a group receiving GP 250
mg/kg once daily as an oral gavage for 4 days for acute experiments (12 rats)
and 12 days for chronic experiments. In addition, atorvastatin was used as a
positive control. Rats were divided into 3 groups (Figure 2). A control group
(12 rats), not receiving active treatment (C) apart from 1% CMC as an oral
gavage, a group treated with P407 1g/kg alone (P407) (15 rats) and a group
treated with 75 mg/kg atorvastatin once daily for 4 days as an oral gavage
(atorvastatin) (9 rats). In the latter 2 groups in each of the above
experiments, hyperlipidemia was induced by injecting rats with P407
intraperitoneally 48 hr prior to blood collection. All groups had free access
to food and water. Pharmacological endpoints measured were TG, TC, HDL
cholesterol, LDL cholesterol and nitrite levels.
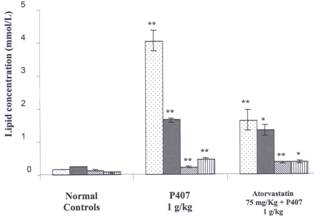
Figure 2: Effects of Atorvastain on Lipids in P407
Hyperlipidemia Three groups are compared; a normal control group received ip saline
injection, a P407 group, received 1 g/kg P407. Rats in these 2 groups were
given an oral gavage of 1% CMC daily for 4 days. In the third group
(atorvastatin 75 mg/kg group) rats were administered atorvastatin 75 mg/kg for
4 days and an injection of P407 1 g/kg ip 48 hr prior to blood collection. Mean
± SEM, n=12 for normal rat group, n=15 for P407 group and n=9 for atorvastatin
group. Lipids measured are triglyceride ![]() total cholesterol l
total cholesterol l ![]() , high density
lipoprotein cholesterol
, high density
lipoprotein cholesterol ![]() and low density lipoproteins
and low density lipoproteins ![]() .*= P < 0.05 and **= P < 0.01 (all relative to normal controls in the
P407 group and relative to P407 group in the atorvastatin group).
.*= P < 0.05 and **= P < 0.01 (all relative to normal controls in the
P407 group and relative to P407 group in the atorvastatin group).
In vitro Effect of Gynostemma pentaphyllun and P407 on Lipoprotein Lipase Activity
To determine the effects of P407 and GP on LPL
in vitro, two rats were anaesthetized with ketamine. Each rat received
an intravenous injection into the tail vein of 1000 IU of heparin in a volume
of 0.3 ml. Two minutes following the heparin injection, blood was drawn using
cardiac puncture. Blood was then pooled centrifuged and plasma frozen until further
experimentation.
To determine the effect of P407 on the activity of LPL
contained in post-heparin plasma from rats, increasing concentrations of P407
(2.5, 5, 10, 20 μM) were incubated (total volume of 0.2 ml) with substrate
enzyme for 15 min at 37ºC. To determine the effect of Gynostemma
pentaphyllum (GP) on the activity of LPL, increasing concentrations of GP
(5, 10, 25, 50, 100 and 200 μg/ml) were incubated in the assay mixture for
15 min at 37ºC. This experiment was performed in the presence or absence of 20
μM P407.
A method to measure LPL established
in 1976 using a stable, radioactive substrate emulsion was employed (12). Fatty
acid-labelled trioleoyglycerol was emulsified by homogenisation in glycerol
with lecithin as detergent. This anhydrous emulsion was stable for 6 weeks.
Substrate solutions for enzyme assay were prepared by diluting the emulsion
with buffer containing serum and albumin. The fatty acid produced on hydrolysis
was isolated in a one-step liquid-liquid partition system (13).
Data Analysis
All data are expressed as the standard error of the mean (± SEM). Comparisons among the control and treatment groups will be made using one-way analysis of variance followed by a Student-Newman-Keuls t-test using the GraphPad Instat statistical program. With all analyses, an associated probability (P value) of less than 5% (P < 0.05) was considered significant.
Results
Hyperlipidemia
The
dose of P407 chosen from a preliminary dose response study in rats was 1 g/kg and the optimum time for
measurement of P407 induced hyperlipidemia was determined to be 48 hours. In
acute studies, lipid values in normal rats were compared with P407 (1 g/kg) treated
rats 48 hr post hyperlipidemia induction. TG levels were increased by 25 fold
(from 1.51 ± 0.1124 mmol/L to 38.24 ± 3.0541 mmol/L), TC levels increased by
more than 6 fold (from 2.52 ± 0.1512 mmol/L to 16.32 ± 0.6321 mmol/L), HDL
levels increased by 1.6 fold from (1.44 ± 0.1511 mmol/L to 2.33 ± 0.1537
mmol/L) and LDL levels increased by 7 fold (from 0.8249± 0.1465 mmol/L to 5.42
± 0.4368 mmol/L). All of these increases in plasma lipids were statistically
significant (P < 0.05).
Effect of Atorvastatin Administered Acutely on P407 Treated Rats
Atorvastatin administered
by oral gavage (75 mg/kg for 4 days) was used as a positive control (Figure.
2). Atorvastatin significantly decreased the elevation of triglycerides;
cholesterol and LDL induced by P407 treatment 48 hr before blood collection,
and also increased HDL levels.
Three
groups are compared; a normal control group received ip saline injection, a
P407 group, received 1 g/kg P407. Rats in these 2 groups were given an oral
gavage of 1% CMC daily for 4 days. In the third group (atorvastatin 75 mg/kg
group) rats were administered atorvastatin 75 mg/kg for 4 days and an injection
of P407 1 g/kg ip 48 hr prior to blood collection. Mean ± SEM, n=12 for normal
rat group, n=15 for P407 group and n=9 for atorvastatin group. Lipids measured
are triglyceride, total cholesterol, high density lipoproteins and low density
lipoproteins.*= P < 0.05 and **= P < 0.01 (all relative
to normal controls in the P407 group and relative to P407 group in the
atorvastatin group).
Effect of Gynostemma pentaphyllum
Administered acutely and chronically on P407 Treated Rats
To determine the acute effect
of GP on lipid levels, rats received GP for 4 consecutive days, after which
hyperlipidemia was induced by injecting P407 48 hr prior to blood collection. GP was found to be effective in significantly
reducing both TG and TC levels after 4 days of pre-treatment at a dose of 250
mg/kg (Figure 3). GP significantly reduced TG levels by 53% (from 38.24 ± 3.0512 mmol/L to
18.04 ± 3.4241 mmol/L) and TC by 10% (from 16.32 ± 0.6348 mmol/L to 14.81 ± 1.5633 mmol/L).
No significant changes were seen
on HDL cholesterol or LDL cholesterol levels but there was a trend towards a
reduction in LDL cholesterol levels.
To determine the effect of a more
chronic pre-treatment of GP on lipid levels, SD rats received GP for 12 days;
subsequently hyperlipidemia was induced by injecting P407 ip 48 hr prior to
blood collection. Chronic administration of GP 250 mg/kg over a twelve day
period significantly reduced TG and TC levels in plasma of P407 treated rats
(Figure 4).
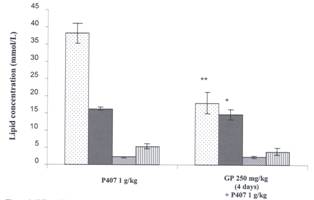
Figure 3: Effect of Gynostemma pentaphyllum on
Lipids in P407 Hyperlipidemia. Acute
administration. Two groups are compared; a P407 group received an oral gavage of 1% CMC
daily for 4 days followed by an ip injection of 1 g/kg P407 48 hr prior to
blood collection. The second group (GP 250 mg/kg) received a daily oral gavage
of GP 250 mg/kg for 4 days and an injection of P407 (1 g/kg) ip 48 hr prior to
blood collection. Mean ± SEM, n=12 for all
groups. Lipids measured are triglyceride ![]() total cholesterol l
total cholesterol l ![]() , high density
lipoprotein cholesterol
, high density
lipoprotein cholesterol ![]() and low density lipoproteins
and low density lipoproteins ![]() .*= P < 0.05 and **= P < 0.01 relative to P407 controls.
.*= P < 0.05 and **= P < 0.01 relative to P407 controls.
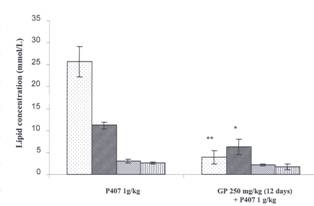
Figure
4. Effect of Gynostemma
pentaphyllum on Lipids in P407 Hyperlipidemia Chronic administration Two groups are compared, a
P407 group which received an oral gavage of 1% CMC daily for 12 days followed
by an ip injection of 1 g/kg P407 48 hr prior to blood collection. The second
group (GP 250 mg/Kg) received a daily oral gavage of GP 250 mg/kg for 12 days
and an injection of P407 (1 g/kg) ip 48 hr prior to blood collection. The data
are expressed as mean ± SEM, n=8 for all
groups. Lipids measured are triglyceride ![]() total cholesterol l
total cholesterol l ![]() , high density
lipoprotein cholesterol
, high density
lipoprotein cholesterol ![]() and low density lipoproteins
and low density lipoproteins ![]() .*= P < 0.05 and **= P < 0.01 relative to P407 controls.
.*= P < 0.05 and **= P < 0.01 relative to P407 controls.
GP significantly reduced TG levels
by 85% (from 25.73 ± 3.5422 mmol/L to 3.98 ± 1.4214 mmol/L), and TC levels by 44% (from 11.27 ± 0.7235 mmol/L to 6.31 ± 1.7044 mmol/L), a 35% reduction in LDL levels (from 2.63 ± 0.4115 mmol/L to 1.72 ± 0.6038 mmol/L), was not statistically significant. No
significant changes in HDL levels were noted.
Effect of Gynostemma pentaphyllum on Plasma Nitrite in P407 Treated Rats
The effect of GP on nitrite levels in plasma was
examined in rats with enhanced nitrite levels induced by P407. Twenty-four h
following injection of 1 g/kg P407 ip to rats, plasma nitrite levels
significantly increased by more than 8 fold (from 8.05 ± 0.02 μM to 68.03
± 6.51 μM). At 48 and 72 h time points, levels increased significantly by
more than 18 fold, to 149.81 ± 3.93 μM and 149.60 ± 5.51 μM,
respectively. In acute studies, where GP 250 mg/kg was administered to SD rats
for 4 days, and P407 (1 g/kg) was injected ip 48 hours prior to plasma collection,
a significant reduction in plasma nitrite levels were observed. Nitrite levels
were reduced by 74% (from 144.63 ± 9.92 μM to 38.21 ± 8.53 μM)
(Figure 5). In chronic studies, where GP 250 mg/kg was fed to SD rats for 12
days, and P407 (1 g/kg) was injected 48 hours prior to plasma collection,
significant reduction in plasma nitrite levels were observed. Nitrite levels
were reduced by 86% (from 144.63 ± 9.92 μM to 20.01 ± 5.93 μM).
Three
groups are compared, a control group received saline injection ip and 1% CMC as
an oral gavage, a P407 group received an oral gavage of 1% CMC daily for 4 days
and an injection of 1 g/kg P407 ip 48 hr prior to blood collection. The third
group (GP 250 mg/kg) received a daily oral gavage of GP 250 mg/kg for 4 days and
an injection of P407 1 g/kg ip 48 hr prior to blood collection. Mean ± SEM, n=12 for all groups. *= P < 0.05 and **= P < 0.01, relative to controls for P407 group
and relative to P407 controls for GP 250 group.
Effect of Gynostemma
pentaphyllum on Lipoprotein lipase
(LPL) Activity in vitro
The in vitro effect of
P407 on LPL enzyme activity was determined. Increasing concentrations of P407
were incubated with the enzyme as described in Methods section. As the dose of
P407 was increased, the activity of LPL was progressively reduced, 50% of the
LPL enzyme activity being inhibited at a poloxamer concentration of
approximately 20 μM. LPL activity
was almost abolished at P407 concentration of 100 μM (Figure 6). The
effect of incubation of various concentrations of GP on LPL enzyme activity was
examined (see Methods section). GP, at concentrations from 5 to 100 μg/ml
in the absence of P407 had no effect on LPL enzyme activity (Figure 7). In the
presence of 20 μM P407 (see Methods section) GP reversed the P407 inhibitory
effect on LPL activity in a dose-dependent manner (Figure 8). At a GP
concentration of 10 μg/ml, LPL enzyme activity increased by 2 fold
compared to the control. Further increases of LPL enzyme activity were observed
as GP concentrations were increased further, however, enzyme activity remained
reached a ceiling effect and remained almost unchanged at 100-200 μg/ml.
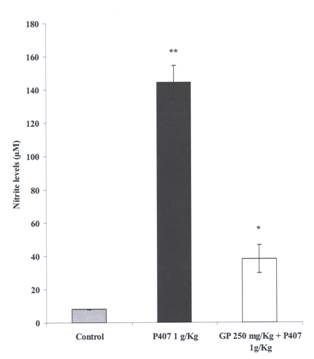
Figure 5: Effect of Gynostemma pentaphyllum On
Plasma Nitrite in P407 Hyperlipidemia. Three groups are compared, a
control group received saline injection ip and 1% CMC as an oral gavage, a P407
group received an oral gavage of 1% CMC daily for 4 days and an injection of 1
g/kg P407 ip 48 hr prior to blood collection. The third group (GP 250 mg/kg)
received a daily oral gavage of GP 250 mg/kg for 4 days and an injection of
P407 1 g/kg ip 48 hr prior to blood collection. Mean ± SEM, n=12 for all groups. *= P
< 0.05 and **= P < 0.01, relative to controls for P407 group and relative
to P407 controls for GP 250 group.
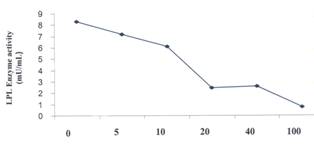
Figure 6: The
Effect of P407 on the Activity of Lipoprotein Lipase. Values are means of duplicate samples assayed
twice. The error bars represent the range of the two replicates.
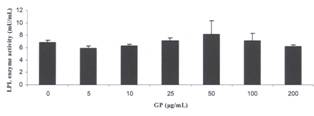
Figure 7 The
Effect of Gynostemma pentaphyllum on Lipoprotein Lipase Activity in the
Absence of P407 Values
are means of duplicate samples assayed twice. The error bars represent the
range of the two replicates,
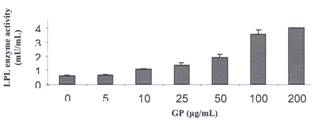
Figure 8. The Effect of Gynostemma
pentaphyllum in the Presence of 20 μM P407 on the Activity of
Lipoprotein Lipase Incubations
were carried out as above in the presence of 20 μM of P407.Values is means
of duplicate samples assayed twice. The error bars represent the range of the
two samples.
Discussion
The first aim of this study was to reproduce
in our laboratory a reliable and reproducible hyperlipidemic rat model,
suitable for rapidly screening the effects of Gynostemma pentaphyllum on
lipid levels. Investigations of antihyperlipidemic effects of xenobiotics have
previously required long-term feeding studies that are often prohibitive to
researchers as they are time consuming and costly. This current model appears
to be reproducible, sensitive cost-effective, and may have applicability for
screening of various sub-fractions of Gynostemma
and other herbs, traditional medicines, nutraceuticals, and other
xenobiotics for anti-hyperlipidemic activity.
The results obtained with the
poloxamer model are comparable to those in the literature (6, 14-19).
Collection of blood at 48 hours post P407 ip injections was considered optimal,
since TG, TC and LDL cholesterol levels were at peak levels. In addition, 1 g/kg
of P407 was considered a suitable dose, since the elevation of lipid levels
were significantly higher than the control, however, they were below the near maximum
levels obtained at 2 g/kg of P407. Thus 1 g/kg P407 and blood collection at 48
hours following induction of P407 were considered suitable conditions for
inducing hyperlipidemia, and sufficient for accurate measurement and of a
suitable magnitude to detect both reductions and increases in lipid levels
following treatment protocols. The
effect of P407 on lipid sub-fractions (LDL and HDL cholesterol) and nitrite
levels was determined in the rat model. P407 increased TG, TC, LDL and HDL cholesterol
and nitrite levels in plasma. However, the fold increase in TG was significantly
greater than all the other lipids measured. This could possibly be due to the
activating effect P407 on endothelial heparin-releasable LPL (19). Furthermore,
the novel finding that injection of P407 ip increased plasma nitrite levels by
more than 10 fold is consistent with hypercholesterolemic patients where in
severe hypercholesterolemic the mean basal value of nitrites was statistically
higher than that of the controls (20). In the P407 model there may be elevated
nitric oxide concentrations due to the increase of LDL consistent with the
hypothesis of a stimulating effect of LDL upon NO endothelial synthesis (20).
These nitrite plasma levels are consistent with what has been demonstrated in
patients with acute coronary heart disease and cholesterolemia. (21) It appears
that significantly enhanced synthesis of plasma nitrites in
hypercholesterolemic patients and the P407 model may involve NO and endothelium-damaging
substances such as LDL. Therefore, this model appears to be a useful model for acute
screening of potential lipid-lowering drugs and also to study the
pathophysiological importance of NO in hyperlipidemia, since hyperlipidemia and
NO are both rapidly induced in this model.
The present study was designed to
examine whether GP would attenuate the hyperlipidemia response observed in P407
treated rats. The effect of 90% pure saponin fraction Gynostemma
pentaphyllum on lipid levels was tested using the P407 model. The dose of Gynostemma
pentaphyllum at 250 mg/kg was found in preliminary studies to be a suitable
dose for producing near maximum decreases in lipid increases and therefore was
used in all studies Gynostemma pentaphyllum was effective following both
acute (4 day) and chronic (12 day) administration to reduce TG and TC levels.
However, chronic treatment yielded significantly greater reductions in both TG
and TC levels.
LDL
cholesterol levels were not reduced by acute administration of GP, and although
LDL cholesterol levels were reduced in the chronic studies by more than 30%, due
to variability statistical significance was not achieved. Our finding in
relation to effect of atorvastatin on LDL cholesterol levels, where LDL cholesterol
declined by 17% in rats, is qualitatively similar to results demonstrating that
LDL cholesterol was reduced by more than 37% in human studies (22). The current
finding is supported by a study conducted by Johnston and co-workers (14),
testing various HMG-CoA reductase inhibitors in P407 induced hyperlipidemia in
C57BL/6 mice. In this study the reduction in TG levels by atorvastatin was more
profound than reductions in TC levels, with TC levels being reduced by 19% and
TG levels by 36%. In another study, with
P407 in normal rats the effect of pravastatin also yielded similar results (18).The
increase in TG seen following P407 ip injection is considered to be mainly due
to the inhibition of TG degradation, due to a direct inhibitory effect on
lipoprotein lipase (LPL) bound to capillary endothelium (7). Hence, the effect on LPL could be a possible
mode of action of Gynostemma pentaphyllum, since it was significantly
effective in reducing TG levels in this model. The hypothesis that GP acts on
LPL activity was therefore examined. LPL is vital in the metabolism of
triglycerides and is involved in several pathological disorders, including
atherosclerosis and obesity. The in vitro
studies demonstrated that Gynostemma
pentaphyllum reversed the inhibitory effect of P407 on heparin-releasable
LPL, whereas it had no effect on LPL in the absence of P407 inhibition.
LPL is organised into two structurally distinct regions,
consisting of a larger amino-terminal domain and a smaller carboxy terminal
end, connected by a flexible peptide. Various studies have shown that the
catalytic triad occurs in a groove that consists of hydrophobic chains of three
sites, with access of the substrate to the active site pocket being blocked by
a polypeptide lid. Binding of the lipoprotein substrate to LPL produces a
conformational change that leads to the opening of the lid and enhancement of
LPL activity (23-25). Our in vitro studies have shown that P407
inactivates LPL enzyme activity when the two are incubated concomitantly. P407
could possibly achieve this by closing this polypeptide lid, hence inactivating
LPL, without denaturing the enzyme. This effect is consistent with previous
literature where both in vitro and in vivo studies indicated that
P407 induced hypertriglyceridemia was due to reversible inactivation of LPL
bound to capillary endothelium (7). The reversal of the inhibitory action of
P407 on LPL by GP could possibly have been achieved by an effect of gypenosides
on opening of the polypeptide lid, previously closed by P407 treatment, hence
activating the enzyme. The current finding of GP activated LPL enzyme activity
is of considerable interest, as LPL plays a central role in the overall
degradation of TG. The fact that GP alone had no effect on LPL enzyme activity
might suggest GP that it does not act directly on the LPL enzyme, but on its
inactivated form. It is tempting to speculate that GP will also activate LPL
inhibited by other metabolic imbalances, but this remains to be determined in
future work. The possible effect of GP on LPL in vivo is the subject of our
ongoing investigations.
Maintaining a balance of
production of nitric oxide is important for the cardiovascular system. At low
levels nitric oxide acts a vasodilator, thereby playing an important role in
the regulation of vessel tone in the cardiovascular system. Lower than normal
production of nitric oxide can be associated with vasoconstriction and may
contribute to atherosclerosis. Overproduction of NO, or cytotoxic NO
metabolites contributes to numerous pathological processes (26). In
atherosclerotic lesions, inflammatory processes up regulate iNOS production and
macrophages, resulting in excessive NO production and vascular damage (27).
Furthermore, excess NO induces oxidation of LDL within the arterial walls and up
regulation of intracellular cell adhesion molecule expression (28). In one study, the direct release of nitric
oxide by gypenosides derived from Gynostemma pentaphyllum was examined in
vitro. In this study, nitric oxide production was observed in bovine aortic
endothelial cells. It was concluded that Gynostemma pentaphyllum
directly stimulated nitric oxide release in these vascular cells (29). More
recently, our laboratory has explored additional mechanisms of action of Gynostemma
pentaphyllum. It was concluded that GP suppresses NO synthesis in murine
macrophages by inhibiting iNOS enzymatic activity to a small extent and
effectively attenuating NF-κB-mediated iNOS expression, implicating these
mechanisms in the GP therapeutic effects (29). In the current study, the new
finding that GP at 250 mg/kg reduced P407 induced elevation of nitrite levels,
in both acute and chronic studies, indicates that GP may also have
anti-inflammatory effects. It is plausible that GP may have cardio protective
or anti-atherosclerotic properties and if this is indeed the case its use in
controlling pathological conditions, including inflammation and cardiovascular
disease warrants further research to examine this hypothesis.
The rat model of P407 hyperlipidemia
may have certain limitations. Lipoprotein metabolism in rat differs from man in
two ways. Firstly, the rat has a highly efficient mechanism for clearance of
chylomicron and VLDL remnants from the circulation; hence rats have lower
levels of LDL. Secondly, the absence of cholesteryl ester transfer proteins
(CETP) in the rat may lead to high levels of HDL, which may act as the main
cholesterol carrier (30). However in one study, CETP-like mRNA was detected by
RNase protection analysis in several rat tissues, namely, heart, skeletal
muscle, adipose tissue and small intestine (31). An interesting observation
from a recent study is the detection of CETP activity and CETP protein in P407
treated rat plasma (19). In this study it was postulated that administration of
P407 to rats causes post-transcriptional up regulation of CETP protein as well
as the corresponding protein activity, which would facilitate increased
transfer of cholesteryl esters between plasma lipoproteins (19). This latter
finding should also be considered in the overall effects of GP in
hyperlipidemia. The testing of saponin activities in vivo is more
relevant than in vitro assays. If saponins have sufficient fat
solubility they can be absorbed unchanged in significant quantities in the
small intestine. However, if saponins are not absorbed they will pass to the
large intestine where gut flora will convert them to sapogenins. Sapogenins
have improved lipid solubility and will be absorbed to a greater extent; hence
in these cases saponin acts as a prodrug with the bioactivity of saponins being
due mainly to their sapogenins. Thus extrapolation of in vitro results
alone for saponins could be unreliable and the use of animal models in these
cases are essential.
Findings in this present study are important for the further characterization of this novel model of hyperlipidemia and in exploring a potentially effective lipid-lowering herbal medicine with traditional use and promising clinical significance. Utilising the poloxamer P407 model, GP was shown to be effective in significantly lowering TG and TC levels, and showed a trend in lowering LDL cholesterol levels in chronic studies. Importantly, it was determined for the first time that plasma nitrite levels were also elevated in this model. These findings are of potential importance in the treatment and/or prevention of cardiovascular diseases. However, more work is needed to investigate possible mechanisms of action of GP. With the growing interest of the Western world in complementary and alternative medicines investigations such as these that scientifically examine traditional beliefs and experience are required and are ever increasingly forthcoming in the literature. Overall, the use of an effective herbal drug to supplement other drug treatments in controlling hyperlipidemia and enhancing cardiac functions could be potentially of clinical value if these models are translatable to human clinical studies and outcomes.
Acknowledgements
Dr. S. Megalli was supported by a post-graduate scholarship from the Faculty of Pharmacy at the University of Sydney. Gynostemma pentaphyllum extract was supplied by Ankang Pharmaceutical Institute of the Beijing University, People’s Republic of China.
References
1. Li L, Lau B. Protection of
vascular endothelial cells from hydrogen peroxide-induced oxidant injury by
Gypenosides, Saponins of Gynostemma pentaphyllum. Phytother Res 7: 299-304, 1992.
2. Cui J, Eneroth P, Bruhn J. Gynostemma
pentaphyllum: identification of major sapogenins and differentiation from
Panax species. Eur J Pharm Sci 8:187-191,
1999.
3. Joo C, Cho Y, Koo J, Kim C, Lee
S. The effect of ginseng saponins on the absorption of rats and cholesterol in
rats. K Biochem J 3:1-17, 1980.
4. Joo
C, Kim D, Koo J. The effect of
ginseng saponin on hypercholesterolemia induced by prolonged cholesterol
feeding in rabbits. K Biochem J 13:51-58,
1980.
5. Wout Z, Maggiore J, Palicharla
P, Johnston T. P407 mediated changes in plasma cholesterol and triglycerides
following intraperitoneal injection to rats. J Parent Sci Tech 46: 192-200, 1992.
6. Palmer W, Emeson E, Johnston T.
P407 induced hyperlipidemic atherogenic animal model. Med Sci Sp Ex 29:1416-1421, 1997.
7. Johnston T,
Plamer W. Mechanism of Poloxamer induced hypertriglyeridemia in rat. Biochem Pharmacol 46: 1037-1042, 1993.
8. Johnston T,
Barker J, Hall D, Jamal S, Plamer W, Emerson E. Potential downregulation of
HMG-CoA reductase after prolonged administration of P407 in C57BL/6 mice. J Cardiovas Pharmacol 34: 831-842,
1999.
9. Allain CC, Poon LS, Chan CS,
10. Bucolo G, David H.
Quantitative determination of serum triglycerides by the use of enzymes. Clin Chem 19: 476-482, 1973.
11. Heuck CC, Erbe I, Mathias D.
Cholesterol determination in serum after fractionation of lipoproteins by
immunoprecipitation. Clin Chem 31: 252-256, 1985.
12 Nilsson-Ethle P, Schotz M. A stable, radioactive substrate emulsion for assay of lipoprotein
lipase. J Lipid Res 17: 536-550, 1976.
13. Belfrage P,
Vaughan M. Simple liquid-liquid partition system for isolation of labelled
oleic acid from mixtures with glycerides. J Lipid Res 10: 341-344, 1969.
14.Johnston T,
Nguyen L, Chu W, Shefer S. Potency of select statin drugs in a new model of
hyperlipidemia and atherosclerosis. Int
J Pharm 229: 75-86, 2001.
15.BrocksDR,
Wasan KM.
The influence of lipids on stereoselective pharmacokinetics of halofantrine:
Important implications in food-effect studies involving drugs that bind to
lipoproteins. J Pharm Sci 91: 1817-1826, 2002.
16. Eliot LA,
Jamali F.
Pharmacokinetics and pharmacodynamics of nifedipine in untreated andatorvastatin-treated
hyperlipidemic rats. J Pharmacol Exp Ther. 1999 Oct;291(1):188-93.
17. Johnston TP,
Palmer WK.
The effect of pravastatin on hepatic 3-hydroxy-3-methylglutaryl CoA reductase
obtained from poloxamer 407-induced hyperlipidemic rats. Pharmacotherapy.
17:342-347, 1997.
18.
Porter J, Carter B, Palmer W. Effects
of pravastatin plasma lipid concentrations in P407 induced hyperlipidemic rats.
Pharmacotherapy 15:92-8, 1995.
19. Wasan K, Kwong M, Goldberg I,
Wright T, Johnston T. P407 mediated alterations in the activities of enzymes
regulating lipid metabolism in rats. J
Pharmacol Sci 6:189-197, 2003.
20. Ferlito S,
Gallina M,
Catassi S,
Bisicchia A,
Di Salvo MM.Nitrite
plasma levels in normolipemic and hypercholesterolemic patients with peripheral
occlusive arteriopathy. Panminerva Med 41:307-309, 1999.
21. Ferlito S,
Gallina M.
Nitrite plasma levels in acute and chronic coronary heart disease. Minerva
Cardioangiol 45: 553-558, 1997.
22. Jacobson T. Clinical context:
Current concepts of coronary heart disease management. Am J Med 110: 3-11, 2001.
23.Mead, J.,
Irvine, S. and Ramji, D. (2002). Lipoprotein lipase: structure, function,
regulation and role in disease. Journal
of Molecular Medicine 80:753-769.
24.Tilbeurgh, V., Roussel, A.,
Lalouel, J. and Cambillau, C. (1994). LPL. Molecular model based on the
pancreatic lipase X ray structure: consequences for heparin binding and
catalysis. Journal of Biological
Chemistry 269:4626-4633.
25.Yang, Gu, Z., Yang, H., Rohde,
M., Gotto, A. and Pownall, H. (1989). Structure of bovine milk LPL. Journal of Biological Chemistry
264:16822-27.
26.Hobbs, A., Higgs, A. and
Moncada (1999). Inhibition of nitric oxide synthase as a potential therapeutic
target, Annual Review of Pharmacology and Toxicology. 39:191-220.
27.Bogdan, C. (2001). Nitric oxide
and the immune response. Natural
Immunity 2:907-916.1.
28.Tanner, M., Bu, X., Steimle, J.
and Myers, P. (1999). The direct release of nitric oxide by gypenosides derived
from the herb Gynostemma pentaphyllum. Nitric oxide 3:359-365.
29.Aktan, F., Henness, S., Roufogalis, B. and
Ammit, A. (2003). Gypenosides derived from Gynostemma
pentaphyllum suppress NO synthesis in Murine macrophages
by inhibiting iNOS enzymatic activity and attenuating NF-κB-mediated iNOS
protein expression. Nitric oxide 8:235-242.
30.Tsi, D. and Tan, B. (2000). The
mechanism underlying the hypocholesterolemic activity of aqueous celery
extract, its butanol and aqueous fractions in genetically hypercholesterolemic
Rico rats. Life Sciences
66:755-767.
31.Jiang, X., Moulin, P., and Quinet, E. (1991). Mammalian adipose tissue and muscle are major sources of lipid transfer protein mRNA. Journal of Biological Chemistry 266:4631-9.
Published by the Canadian Society for Pharmaceutical Sciences.
Copyright © 1998 by the Canadian Society for Pharmaceutical Sciences.
CSPS Home | JPPS Home | Search | Subscribe to JPPS