J Pharm Pharmaceut Sci
(www.cspscanada.org) 8(3):552-557, 2005
The effect of structured triglycerides on the
kinetic stability of total nutrient admixtures.
Judit Balogha, Júlia Bubenika,
Judit Dredánb, Ferenc Csempeszc, Dorottya Kissa,
Romána Zelkóa
aUniversity Pharmacy Department of Pharmacy Administration,
Semmelweis
University,
Budapest, Hungary
bDepartment of Pharmaceutics, Semmelweis
University, Budapest, Hungary
cDepartment of Colloid Chemistry, Eötvös
L. University,
Budapest, Hungary
Received July 19, 2005; Revised September 22,
2005; Accepted September 28, 2005, Published October 5, 2005
PDF
Version
Abstract
Purpose The physical stability of two
types of total parenteral nutrient (TPN) admixtures was studied as a
function of storage time and temperature. One of them contained only structured
triglycerides and the other exclusively long-chain triglycerides as lipid
components.
Methods Droplet size of the mixtures was followed by photon
correlation spectroscopy for 10 days. Zeta potential and dynamic surface
tension measurements were carried out to evaluate the possible changes in the
charge and interfacial surface tension of the emulsion droplets during the
storage. pH values were monitored in order to follow the possible decomposition
processes in the course of storage.
Results
Droplet size of emulsions prepared with lipids containing exclusively
long-chain triglycerides showed remarkable increase after 4 days of storage in
contrast with that of the mixtures containing structured lipids.
Conclusions The obtained results indicate that besides the
advantageous metabolic effects of structured triglycerides, their application is recommended to improve
the physical stability of TPN
admixtures.
Introduction
Pharmaceutical-grade intravenous lipid emulsions are complex dispersions
of oil droplets that have been carefully homogenized
to produce high-quality dispersions, safe for intravenous administration, with
particles of a mean dimension approximately 300 nm in diameter. This mean lipid
droplet size is within the typical range of the dimensions of endogenous
chylomicrons (80 – 500 nm), and the formulations are manufactured in this way to
behave in a similar manner with respect to their metabolic fate (1, 2).
Intravenous lipid emulsions are systems of high
physicochemical stability, thus their shelf life can be as long as 24 months when
stored at 25°C. Washington et al. investigated the stability of Intralipid and
Ivelip infusions, and found that the emulsions could be considered stable even
after being subjected to accelerated tests such as autoclaving (3).
As total nutrient admixtures are solutions comprising 60 or more
chemical species in a single container, destabilization of lipid emulsions
often occurs, which results in reversible aggregation or flocculation of the
droplets, followed by irreversible coalescence after relatively short storage intervals
(4, 5). When the volume-weighted percentage of fat at a threshold of five
μm exceeds 0.4% of the total lipids present, danger of fat embolism
reaches a critical level (6).
Several methods can be used for the assessment of
physical stability of lipid emulsions, including particle size analysis via
photon correlation spectroscopy, light obscuration, laser diffraction or
microscopy (7-9). While these methods can follow physical changes,
zeta-potential and pH measurements are able to indicate chemical processes that
take place along with storage. Dynamic surface tension measurements can provide
additional information concerning the physicochemical processes that take place
on the surface of the lipid droplets.
Electrolytes added to the mixtures affect the
stability of emulsions via alteration of the zeta-potential caused by the negatively
charged head groups of phospholipids used as emulsifying agents in most
parenteral lipid emulsions (4). It has been shown in previous works available
in the literature that the type of triglycerides in the lipid component also
influences the stability of all-in-one mixtures. It has been reported that pure
long-chain triglyceride (LCT)-based admixtures degrade to a
much greater extent than those containing medium-chain triglycerides
(MCTs) and LCTs. However, the stabilizing effect of MCTs is lost when physical
mixtures of MCTs and LCTs are made extemporaneously from two separate starting
emulsions (10, 11).
Structured triglycerides (STs), in which both
medium-chain fatty acids and long-chain fatty acids are esterified to the same
glycerol molecule, have positive metabolic effects, which make them competitive
or even more efficient as an energy source compared with conventional fat
emulsions (12). They are assumed to provide a higher oxidation
rate, faster clearance from blood, improved nitrogen sparing, and less of a
tendency to accumulate in the reticuloendothelial system compared with LCT
emulsions (13, 14).
Although the advantageous metabolic effects of STs have been
widely studied, their impact on the physicochemical properties of TPN mixtures has not been clarified yet. The purpose of the present work
was to study the effect of STs on the kinetic stability of total parenteral
admixtures in comparison with lipids containing exclusively LCTs.
Materials and methods
Table 1
summarizes
the composition and
Table 2 comprises the total ionic concentrations of the
prepared TPN (Total Parenteral Nutrition) mixtures.
Table 1 Composition of the
TPN mixtures
|
Compounds
|
Quantity
(ml)
|
|
TPN mixture 1
|
TPN mixture 2
|
|
Infusio glucosi 40% (University
Pharmacy of the Semmelweis University, Budapest)
Glucose anhydrate 400 g
Hydrochloric acid 0,1N 1,000 ml per 1000 ml solution
|
500
|
500
|
|
Elektrolit A (University
Pharmacy of the Semmelweis University, Budapest)
Sodium chloride
4.675 g
Potassium chloride
3.727 g
Magnesium sulfate
cryst 2.00 g
Aqua destillata pro
inj. ad 100.0 ml
|
100
|
100
|
|
Aminoven 10% 500ml inf. (Fresenius Kabi AB Sweden)
L-isoleucine 5.00
g, L-leucine 7.40 g, L-methionine 4.30 g, L-lysine-acetate 9.31 g (=6.6 g
L-lysine), L-phenylalanine 5.10 g, L-threonine 4.4 g, L- tryptophane 2.00 g,
L-valine 6.20 g, L-arginine 12.0g, L-hystidine 3.00 g, L-alanine 14.0 g,
Glycine 11.0 g, L-proline 11.2 g, L-serine 6.50 g, L-tyrosine 0.40 g, Taurine
1.00 g per 1000 ml solution
Total amino acid content 100.0 g/l
|
1000
|
1000
|
|
Intralipid 20% inf. (Fresenius Kabi, Germany GmbH)
Soybean oil: 200 g
Purified egg phospholipids: 12 g
Glycerol (anhydrous) (Ph Eur): 22.0 g
Water for injection to 1000 ml
|
500
|
-
|
|
Structolipid 20% inf. (Fresenius Kabi, Germany GmbH)
Structured triglycerides: 200 g
Purified egg phospholipids: 12 g
Glycerol (anhydrous) (Ph Eur): 22.0 g
Water for injection to 1000 ml
|
-
|
500
|
Table 2 Ionic concentrations of the prepared TPN mixtures
Compounds
|
Concentration (mol/dm3)
in the TPN mixture
|
|
Na+
|
0.0380
|
|
K+
|
0.0238
|
|
Mg2+
|
0.0039
|
|
Cl-
|
0.0618
|
|
SO42-
|
0.0039
|
Preparation of
the TPN Mixtures
The blending of the
compounds of various TPN systems was carried out in a
laminar airflow box (Relatec,
Germany) under
vacuum. The final preparations consisted of four different types of basic
ingredients: amino-acids, carbohydrates, electrolytes
and lipids. The blending of the compounds was carried out
under vacuum in a completely closed system. First, half of the volume of the
Glucose inf. was sucked into the plastic bag through
one of the plastic tubes that was connected to the bag. The electrolytes were added to the remained volume of Glucose infusion and then
sucked into the plastic bag. Next, amino-acids
were blended to the obtained solution. The last step was the addition of lipids
to the solution by sucking the lipid emulsions into the plastic bag. The right
order of the blending assured the homogeneity of the TPN mixtures.
Storage of the Prepared TPN Mixtures
The TPN mixtures
were stored at 2-8 °C and 37 ± 0.5°C temperatures
for 10 days.
Photon Correlation Spectroscopy
The particle size
distribution of emulsions of two different compositions was
examined before storage and after 4, 7 and 10 days. Dynamic light
scattering measurements were carried out for checking
the kinetic stability of the TPN emulsions. The apparatus (Brookhaven
Instruments Corporation) used consisted of a BI-200SM goniometer and a
BI-9000BO Correlator. An Argon-Ion Laser (Omnichrome 543 AP) set to the
wavelength of 488 nm was applied as a light source.
The homodyne autocorrelation function in channel 238 was determined at real
time mode using logarithmic timescale with a range of 1-200000 μs.
Detector angle was set to 90.0 deg., and the gap was 100 μm. Before the measurements,
the emulsions were diluted to reach the appropriate
count rate value. The time of measurement was 180s. Six parallel examinations were carried out on each sample (four different samples –
according to the temperature of storage and the type of lipid emulsion used for
the preparation). Data were evaluated assuming an
exponential distribution of the emulsion particles. The results were plotted as intensity vs. particle size of the emulsion
droplets.
Zeta-potential Measurements
Laser Doppler-electrophoresis (LDE) was used
for investigating the surface-electric properties of the emulsion droplets. Measurements
were carried out before storage and after 4, 7 and 10
days. For electrically charged particles moving in response to an applied
electric field, a correlation function of laser Doppler-shift was measured with
a Malvern Zetasizer 4 apparatus at 25 ± 1°C (Malvern Instruments, UK),
and the resulting frequency spectrum was translated to electrophoretic
mobility. Using an AZ 104 type cell, 5 mobility measurements were ordinarily
done on each sample (four different samples – according to the temperature of
storage and the type of lipid emulsion used for the preparation) in cross beam
mode. The zeta potential (z) of the particles
was calculated from the mobility measurements, using the Smoluchowsky formula.
pH Measurements
pH values of the TPN
mixtures were measured right after preparation and after 1, 4, 7 and 10 days of
storage with a Radelkis OP-300 electroanalytical analyser.
Dynamic Surface Tension Measurements
The examinations were carried out
on the day of preparation and after 1, 4, 7 and 10 days. The surface tension of
emulsions was determined by dynamic method, applying
Du-Noüy ring and Wilhelmy plate operations of a computer-controlled KSV Sigma
70 tensiometer (KSV Sigma 70, RBM-R. Braumann GmbH, Germany)
at 25°C
±
0.5 °C.
The method determines the maximum mass of liquid pulled from the surface by
lifting the specified solid (e.g. ring or plate). The force (f) measured on the
electric balance is necessary for lifting out and pushing down the solid
measuring device from the surface of the liquid.
The contact angle can be calculated
from the extrapolated buoyancy slope:
cos q =
f/pgLV (1)
where q is the contact
angle, f is the force measured on the balance, p is the measured plate
perimeter and gLV is the surface tension (interfacial free
energy between the liquid and vapour) of the examined liquid. 3 parallel measurements were carried out on all four kinds
of samples.
Statistical Evaluation
Zeta-potential
values of the two kinds of mixtures at different temperatures and storage
intervals were compared using the two-sample t-test
assuming equal variances. In this case, the comparison was
made between Intralipid-containing infusions and Structolipid-containing
ones. Surface tension values measured after different storage intervals were compared via the paired two-sample t-test for both
kinds of mixtures. The comparison was made between data obtained right after
preparation and after 1, 4, 7 and 10 days,
respectively.
The statistics were calculated using Microsoft
Excel 2002.
Results and discussion
Figures 1-2 illustrate the average droplet size of the two different TPN
emulsions at different storage temperatures. The mean droplet size of
Structolipid 20% before mixing with the other components was
reported to be 276 nm (9) and proved to be between 300-400 nm in the admixtures
at zero time. The results unambiguously indicate that the average
droplet size of emulsions containing structured triglycerides did not
significantly change during the examined storage period. In contrast, the
droplet size of emulsions prepared with lipids containing exclusively
long-chain triglycerides, showed remarkable increase even after 4 days of
storage. The mean droplet size of Structolipid 20% before mixing with the other
components was reported to be 276 nm (9) and proved to be
between 300-400 nm in the admixtures at zero time. As commercially
available lipid emulsions can be stored for 24 months, these findings confirm
the fact that the additives mixed to these systems negatively influence their
stability.
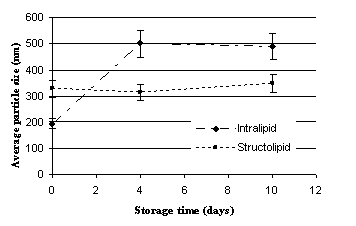
Figure 1 – Effect of storage time on the average droplet size of
the prepared TPN systems; Storage temperature: 2-8 0C
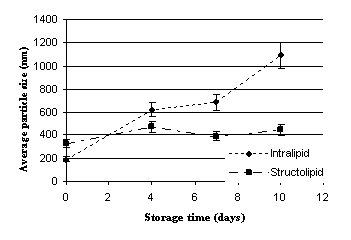
Figure
2 – Effect of storage time on
the average droplet size of the prepared TPN systems; Storage temperature: 37 ± 0.5 0C
Table 3 shows the zeta-potential values of the two
mixtures after storing at different temperatures for 10 days. Such values of
intravenous lipid emulsions can be found in the literature and are in the range
of -40 to -50 mV (4), which shows remarkable increase (i.e. weaker repulsive
forces between the droplets) in the admixtures. No significant difference could be observed between the two kinds of compositions at
zero time, which suggests that their initial stability can be considered
equivalent. p values indicate significant differences
between the two compositions after 4 and 7 days of storage. The more negative
zeta-potential values of the mixture containing structured lipids confirm the
results of the particle-size analysis, i.e. the enhanced stability of the
system prepared with Structolipid. After 10 days, the zeta-potential values can be considered equivalent again, which is probably the
result of the starting destabilization process of the composition containing
structured lipids.
Since the ionic concentration of the two TPN emulsions was equal and pH
values measured in the course of storage (Table 4) did not present remarkable
changes, the lower physicochemical stability of emulsions prepared with LCTs
can not be ascribed to electrostatic effects or chemical decomposition.
Very likely, the formation of a “mixed” interfacial
layer formed from the medium and long chain fatty acids in case of structured
triglycerides is responsible for the more efficient stabilization. The latter could be tracked by the different interfacial surface
structure of the dispersed droplets.
Table 3 Electrokinetic
characteristics of different TPN emulsions (average of 5 parallel measurements,
± S.D.; α = 0.05)
|
Storage time (days)
|
Temperature (°C±0.5°C )
|
Zeta potential (mV)
|
p
|
|
Intralipid
|
Structolipid
|
|
0
|
25
|
-2.2 ± 0.10
|
-1.9 ± 0.35
|
> 0.05
|
|
4
|
2-8
|
-2.4 ± 0.15
|
-2.9 ± 0.15
|
< 0.05
|
|
4
|
37
|
-3.0 ± 0.40
|
-4.1 ± 0.40
|
< 0.05
|
|
7
|
2-8
|
-1.7 ± 0.05
|
-2.9 ± 0.60
|
< 0.05
|
|
7
|
37
|
-2.7 ± 0.60
|
-3.9 ± 0.15
|
< 0.05
|
|
10
|
2-8
|
-2.0 ± 0.20
|
- 2.9 ± 0.90
|
> 0.05
|
|
10
|
37
|
-3.3 ± 0.05
|
- 2.9 ± 0.40
|
> 0.05
|
Table
4 pH values of the mixtures
before and after storage under different conditions (average of 3 parallels, ±
S.D.)
|
Storage time (days)
|
Temperature (°C±0.5°C)
|
pH
|
|
Intralipid
|
Structolipid
|
|
0
|
25
|
5.8 ± 0.1
|
5.8 ± 0.2
|
|
1
|
2-8
|
5.7 ± 0.1
|
5.9 ± 0.1
|
|
1
|
37
|
5.7 ± 0.2
|
5.7 ± 0.1
|
|
4
|
2-8
|
5.9 ± 0.2
|
6.0 ± 0.1
|
|
4
|
37
|
5.8 ± 0.1
|
5.8 ± 0.2
|
|
7
|
2-8
|
5.9 ± 0.1
|
5.9 ± 0.2
|
|
7
|
37
|
5.7 ± 0.1
|
5.7 ± 0.3
|
|
10
|
2-8
|
5.9 ± 0.2
|
5.9 ± 0.1
|
|
10
|
37
|
5.7 ± 0.1
|
5.7 ± 0.2
|
The surface tension values measured by the Wilhelmy
plate operations are summarized in
Table 5. The
measured surface tension of purified water was 58.81 ±0.113
mN/m. The surface tension values determined with Du-Noüy ring correlated well
to values measured by the plate method, but the latter resulted in higher
accuracy. As it can be seen in Table 5, the obtained surface
tension remained almost constant within the examined storage intervals in the
case of admixtures containing the structured lipid component – indicating a
more stable interfacial surface structure. p values
indicate significant difference compared to zero time only after 10 days of
storage at 2-8°C. In contrast, the surface tension of emulsions containing
exclusively long-chain triglycerides remarkably decreased during storage
referring to the interfacial structural changes. In the case of the sample
stored at 37°C, a significant change could be observed
after 4 days. Although further studies are needed to
elucidate the mechanism of the (steric) stabilization, dynamic surface tension
measurements can be recommended as sensitive means for the stability tests of
intravenous lipid emulsions.
Table
5 Surface tension values of different TPN
emulsions stored under different conditions (average of 3 parallels, ± S.D.). p refers to the comparison of the surface tension values
with the corresponding values at zero time (α = 0.05).
|
Storage time (days)
|
Surface tension (mN/m)
|
|
Structolipid
|
Intralipid
|
|
2-8°C
|
p
|
37°C
|
p
|
2-8°C
|
p
|
37°C
|
p
|
|
0 (25°C)
|
30.49 ±0.384
|
-
|
30.49 ±0.326
|
-
|
33.48 ±0.620
|
-
|
33.48 ±0.408
|
-
|
|
1
|
30.90 ±0.846
|
>0.05
|
30.28 ±0.846
|
>0.05
|
33.06 ±0.887
|
>0.05
|
31.53 ±0.725
|
<0.05
|
|
4
|
30.39 ±0.164
|
>0.05
|
30.47 ±0.095
|
>0.05
|
28.12 ±0.867
|
<0.05
|
24.33 ±0.826
|
<0.05
|
|
7
|
30.19 ±0.503
|
>0.05
|
30.47 ±0.437
|
>0.05
|
26.16 ±0.584
|
<0.05
|
26.36 ±0.500
|
<0.05
|
|
10
|
32.17 ±0.342
|
<0.05
|
31.50 ±0.425
|
>0.05
|
27.58 ±0.872
|
<0.05
|
27.06 ±0.537
|
<0.05
|
The findings of this study are in good correlation
with the results of Driscoll et al. concerning the stability of all-in-one
admixtures containing MCTs and LCTs previously mixed in a single emulsion or
added separately to the mixtures (10). As it was reported,
separate droplets of MCTs and LCTs resulted in impaired physicochemical
stability compared to the ones containing both kinds of triglycerides. In the
case of structured lipids, both medium and long chain fatty acids can be found in the starting lipid emulsion, leading to a
favourable interfacial location of structured triglycerides.
The clinical significance of the present study lies in
the recognition that with the application of total nutrient admixtures
containing structured lipids, the incidence of fatal consequences of parenteral
nutrition (e.g. fat embolism) could be decreased.
Conclusions
Kinetic stability of two total nutrient
admixtures prepared with different lipid emulsions (Intralipid and
Structolipid, respectively) was tracked for 10 days
with an array of physicochemical methods. Besides the commonly applied
techniques such as photon correlation spectroscopy and zeta-potential
measurements, dynamic surface tension studies can contribute to the evaluation
of the stability of TPN mixtures. In addition to the advantageous metabolic
effects of structured triglycerides, their application is recommended also to
improve the physical stability of TPN
admixtures, which could decrease the risk of fat embolism in the clinical
practice.
References
1. Carpentier
Y.A.; Simoens C.; Siderova V.; El Nakadi I.; Vanweyenberg V.; Eggericky D.; Deckelbaum
R.J. Recent developments in lipid emulsions: Relevance to intensive care. Nutrition,
13: 73S-78S, 1997.
2. Driscoll
D.F. Physicochemical assessment of total nutrient admixture stability and safety:
Quantifying the risk. Nutrition, 12: 166-167, 1997.
3. Washington
C., Koosha F., Davis S.S. Physicochemical
properties of parenteral fat emulsions containing 20% triglyceride; Intralipid
and Ivelip. J Clin Pharm Ther, 18(2): 123-131, 1993.
4. Washington
C. Stability of lipid emulsions for drug delivery. Adv Drug Deliver Rev, 20:
131-145, 1996.
5. Ball P.A.
Methods of assessing stability of parenteral nutrition regimens. Curr Opin Clin
Nutr, 4: 345-349, 2001.
6. Driscoll
D.F.; Bhargava H.N.; Li L.; Zaim R.H.; Babayan V.K.; Bistrian, B.R.
Physicochemical stability of total nutrient admixtures. Am J Health Sys Pharm,
52: 623-634, 1995.
7. Sayeed
F.A.; Tripp M.G.; Sukumaran K.B., Mikrut B.A.; Stelmach H.A.; Raihle J.A.
Stability of various nutrient admixture formulations using Liposyn II and
Aminosyn II. Am J Hosp Pharm, 44(10): 2280-2286, 1987.
8. Bullock
L.; Fitzgerald J.F.; Walter W.V. Emulsion stability in total nutrient
admixtures containing a pediatric amino acid formulation. J Parent Ent Nutr,
16: 64-68, 1992.
9. Driscoll
D.F., Etzler F., Barber T.A., Nehne J., Niemann W., Bistrian B.R.
Physicochemical assessments of parenteral lipid emulsions: light obscuration
versus laser diffraction. Int J Pharm, 219: 21-37, 2001.
10. Driscoll
D.F., Nehne J., Peterss H., Franke R., Bistrian B.R., Niemann W. The influence
of medium-chain triglycerides on the stability of all-in-one formulations. Int
J Pharm 240: 1-10, 2002.
11. Driscoll
D.F.; Nehne J.; Peterss H.; Klütsch K.; Bistrian B.R.; Niemann W.
Physicochemical stability of intravenous lipid emulsions as all-in-one
admixtures intended for the very young. Clin Nutr, 22(5): 489-495, 2003.
12. Hyltander A.; Sandstrom R.; Lundholm K. Metabolic effects of
structured triglycerides in humans. Nutr Clin Pract, 10(3): 91-97, 1995.
13. Sandstrom R.; Hyltander A.; Korner U.; Lundholm K. Structured
triglycerides to postoperative patients: a safety and tolerance study. J Parenter Enteral Nutr, 17(2): 153-157, 1993.
14. Tso
P.; Lee T.; DeMichele S.J. Randomized structured triglycerides increase
lymphatic absorption of tocopherol and retinol compared with the equivalent
physical mixture in a rat model of fat malabsorption. J Nutr, 131:2157-2163, 2001.
JPPS Contents
Published by the Canadian Society for
Pharmaceutical Sciences.
Copyright © 1998 by the Canadian
Society for Pharmaceutical Sciences.
http://www.cspscanada.org/
CSPS Home |
JPPS Home |
Search |
Subscribe to JPPS

