The samples were treated with an UV-B dose of 0.25
J/cm2 which corresponds approximately with the 10 fold of
the minimal erythema dose (MED) for normal pigmented (type II in the skin type
classification) people [25]. This high dosage was required to provoke stress
conditions.
Thiobarbituric
acid assay
The thiobarbituric acid test
is a quantitative assay for the detection of MDA, and is the most frequently
used technique to determine lipid peroxidation products.
In this study, the Buege-Aust method of the TBA assay
was applied [26]. Two millitres of a stock TBA reagent containing 15 %
(w/v) trichloroacetic acid in 0.25 M HCl and 0.37 % (w/v) thiobarbituric acid
in 0.25 M HCl were added to 1.0 ml of the UV-B treated sample. After heating at
90° C for 15 minutes and cooling , the red
TBA:MDA-complex (2:1) appears and allows fluorescence measurement. An HPLC
system (Merck-Hitachi, Darmstadt,
Germany)
equipped with an autosampler AS-4000A, interface D-6000A, pump L-6200A,
UV-VIS-Detector L-4250 and a fluorescence detector F-1080 was used to quantify
the pigment. A reversed phase column (LiChrospherâ 100, RP 8, particle size 5 µm) was used
with a mobile phase of methanol/water (30/70). The excitation wavelength was
515 nm and the emission measurement was performed at 555 nm.
A calibration curve was generated using MDA which was
formed from malondialdehyde-bis-(dimethylacetal) under acidic conditions.
Statistical
analysis
All data shown
represent the mean values ± SD of the measurements (n = 6).
Statistical analysis of the effects of the different test substances or
extracts on the TBA-RP concentration after UV-B irradiation was performed using
a one-way ANOVA.
In all cases, post-hoc comparisons of the means
of individual groups were performed using Dunnett`s multiple comparison test. A
significance level of P < 0.05 (*) between groups was accepted as being
statistically significant. All calculations were performed using GraphPad Prism
2.0 (GraphPad Software Inc., San Diego,
CA, USA).
Results and
discussion
Development of suitable model systems for
an antioxidative screening
In search for
novel, unknown antioxidants for topical application, a suitable model was
required. There were two standards which these model systems should meet.
Firstly, these systems should be simple to avoid an overlapping of effects
which would make data interpretation difficult or impossible. Secondly, the main
properties of the stratum corneum intercellular lipid matrix should be
present to assure
similarity to the horny layer of human skin. A simple system
containing only one stratum corneum lipid was used for the experiments.
Furthermore, a complex system which is similar to the real horny layer of the
skin was generated by stratum corneum lipid addition.
Substances
belonging to the physiological horny layer lipid matrix were chosen as model lipids for the
experiments. Chol, LLA and DPPC as a liposome generator were used for system modelling.
The test
substances had to have at least one of the following properties to be included
in the screening study:
a)
the exact mechanism of action of the substance is not
currently known in detail,
b)
the substance is present in skin physiologically,
c)
the substance may act as an antioxidant due to its
structural properties,
d)
the incorporation into modern semisolid formulations
for topical application is possible from a pharmaceutical and technological
point of view,
e)
although the substance has
another main effect, antioxidative behaviour may be assumed and therefore screening is worthwhile.
Thiobarbituric
acid assay
Drugs
Figure 1 depicts
the results of the thiobarbituric acid assay of the simple stratum corneum
lipid model system when adding different drugs as test substances. The effects
of 100 µM screening substance on a LLA dispersion are
shown. Figure 1A shows surprising effects for ascorbic acid and amantadine.
When adding ascorbic acid the secondary lipid peroxidation products (measured
as malondialdehyde units) are significantly increased.
Conflicting data
concerning the interactions of vitamin C with ROS has lead to controversy in
the literature. It is reported that ascorbic acid may have both antioxidant and
pro-oxidant properties [27,28]. Genotoxic effects were
found and this was suggested to be a result of the ability of ascorbic acid to
decompose lipid hydroperoxides to DNA damaging secondary products [29].
Recently, we have shown that ascorbic acid exhibits
concentration dependent pro-oxidative effects on lipid model systems of
differing complexity. The molecular mechanism of ascorbic acid degradation after its
lipid damaging action has been demonstrated by mass spectro-metry and detailed
studies regarding the redox properties of ascorbic acid were carried out by EPR
investigations [16]. Firstly, ascorbic acid reduces FeIII ions. More
FeII ions are available and the Fenton reaction can run at a higher level
leading to more peroxidized lipids. Following the results of Lee et al.
[29], more substrate for ascorbic acid to decompose is in the system. The
amount of cell toxic aldehydes is increased which may be the reason for the
increased levels of the TBA assay.
The drug amantadine, used for Parkinson disease
treatment, was included in the screening experiments as well. The lipid
protective effect of amantadine in this study can be explained by a Schiff base
reaction where the amino group of amantadine reacts with the carbonyl group of
malondialdehyde resulting in the formation of
azomethine. Hence, the analyte is masked and cannot be quantified
properly by the TBA assay. This corresponds with the results of
Albrecht-Goepfert et al. [30]. Several aminoadamantane derivatives have
been tested for their influences on ROS. Neither radical scavenging nor singlet
oxygen quenching was observed. On the other hand, topical application of
amantadine could be of advantage because skin damaging carbonyls can be
neutralised this way.
Figure 1B depicts the protective effects of the non-steroidal
antiphlogistic drug, bufexamac and the amino acid tryptophan. While antioxidant
effects of the melatonin precursor, tryptophan, have already been described in
the literature [31], those properties of bufexamac are a new finding. Recently,
we have shown that bufexamac exhibits antioxidative effects on both lipid model
systems and HaCaT keratinocytes. EPR spectroscopy was used for detailed studies
regarding the radical scavenging properties of bufexamac. The molecular
mechanism of the bufexamac fragmentation and degradation after UV exposure has
been demonstrated by mass spectrometry [18].
Some of the often described naturally- occurring
antioxidants, such as beta-carotene, ubiquinone and uric acid [32],
surprisingly showed no effects in this study (Figure 1B).
The significant lipid protective effects of melatonin,
melanin, propranolol and hyaluronic acid on the LLA dispersion after UV
irradiation are demonstrated in Figure 1C. The results for melanin may be due
to its filtering properties of UV radiation. This is because the complex
tyrosine derivative, after its in vivo synthesis in the melanocytes of
the skin, is responsible for the protection of the DNA. It is also considered
to be a radical scavenger [33]. A very interesting result is the inability of
substances usually utilised in sunscreens to protect the lipids from UV induced
peroxidation. The substances which were used to represent these UV filter
molecules were the following: dibenzoylmethane, 7H benzimidazol and
ethyl-p-aminobenzoate. All three chemicals were ineffective in providing
significant lipid protection after UV irradiation in this study.
Antioxidative properties of propranolol [34] and
melatonin [35,36] can be found in the literature.
Melatonin has been found to protect human skin from UV induced erythema in an in
vivo study when topically used on its own or applied in combination with
vitamin E and C [37].
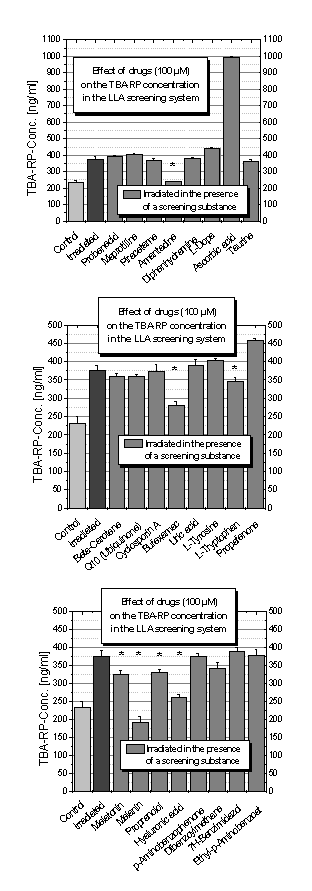
Figure 1. Concentration of thiobarbituric acid reaction products
(TBA-RP Conc.) and influence of ultraviolet irradiation and different test
drugs (100 µM each) in the LLA screening system.
The
antioxidative properties of hyaluronic acid were recently shown to be due to
transition metal scavenging. The fragments of hyaluronan with smaller molecular
weights resulting from enzymatic digestion showed antioxidant properties as
well [17].
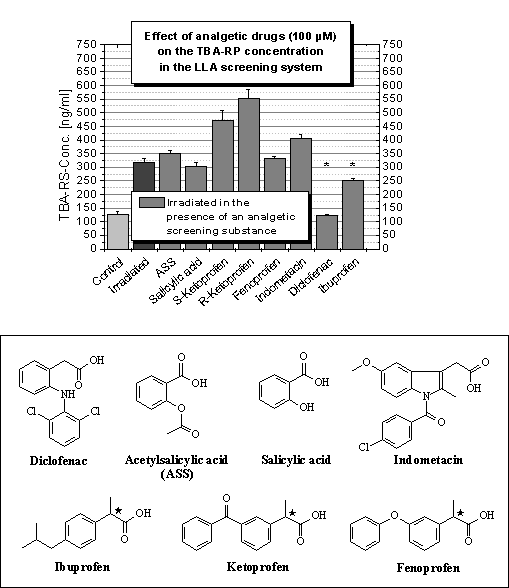
Analgesics
Due to the
bufexamac results more analgesics were tested showing protective effects of diclofenac
[38] and ibuprofen [39] together with pro-oxidative influences of both
ketoprofen enantiomers (Figure 2A). The structures of all analgesics tested are
depicted in Figure 2B. The properties of ketoprofen should be known because the
instruction leaflet of ointments containing this substance warns about sunlight
exposure and other UV treatment of the skin when using the formulation [40].
The phototoxicity of the ketone after UV irradiation of the molecule has been
explained by the generation of hydrogen peroxide determined by capillary
electrophoresis experiments [41]. Its analogue fenoprofen showed no effects on
the amount of MDA after UV irradiation and for ibuprofen lipid protective
effects were measured. Therebye, the phototoxic properties of ketoprofen must
be connected with its benzophenone structure (Figure 2B). Acetyl-salicylic acid
and salicylic acid failed to protect lipids from oxidative damage and the
addition of indometacin lead to a slight augmentation of the MDA amount after
UV stressing.
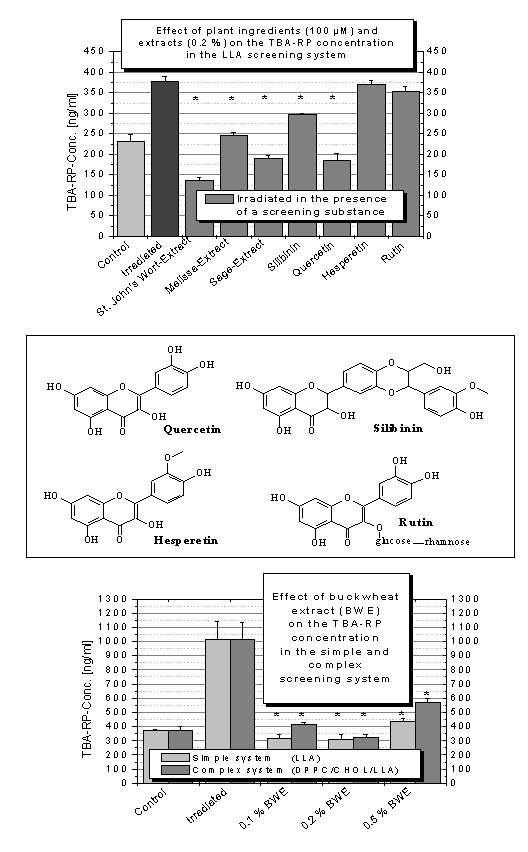
Plant ingredients and plant extracts
The properties
of flavonoids to act as naturally occurring antioxidants in food and their
potential role in the prevention of chronic diseases are well-known and have
been reviewed [42,43]. The antioxidative behaviour of these polyphenolic plant
secondary metabolites is attributed to reductive, radical scavenging, singlet
oxygen quenching and transition metal chelating properties of flavonoids.
Recently Kessler et al. [44] published a detailed study of the
relationship between the structure and the radical scavenging potential of the
flavonoids and used this data to explain the anti- and pro-oxidant activity
measured during their experiments with rutin and other quercetin derivatives.
Among the four flavonoids tested in our study only the
St. Mary`s thistle ingredient
silibinin and the flavone quercetin
showed a lipid protecting property when added to the samples before UV
irradiation. Hesperetin and rutin did not act antioxidatively in this study
(Figure 3A).
All the plant extracts used in this antioxidant
screening showed helpful effects in decreasing the amount of UV irradiation
induced lipid peroxidation. The extracts of St. John`s Wort, melissa and sage
(Figure 3A) as well as buckwheat extract acted as protectors. (Figure 3C). The properties of the St. John`s Wort extract
are due to its flavanoid and phenolic acid content [47] as well as the
antioxidative behaviour of the sage extract [48]. The performance of melissa
extract is explained by the polyphenols which the plant synthesises i.e.
rosmarinic acid, chlorogenic acid, ferulic acid and caffeic acid [49,50]. The buckwheat extract effects on the lipids are
demonstrated by showing the results of both simple and complex screening
systems. The flavonoid fraction of Fagopyrum esculentum contains several
flavonoids such as the aglycone quercetin which showed an antioxidative effect when tested as
a single flavonol. The antioxidative properties of buckwheat
extract towards oxidative stress has been shown in various in vivo
and in vitro systems [51].
Remarkably the TBA-RP concentration levels after
irradiation were significantly below the measured value of the non -UV
irradiated control using St.John’s Wort and the buckwheat samples (0.1%. 0.2%,
respectively). This means that these extracts are able to prevent UV induced
lipid peroxidation and furthermore have the ability to reduce the content of
already existing aldehydic lipid peroxidation secondary products. This may be
explained by a UV catalysed carbonyl reaction of MDA with some of the other
plant extract ingredients, such as tanning agents, bitter constituents or
essential oils.
Considering topical administration of the substances
found to be lipid protective one has to bear in mind the phototoxic potential
of the St. John`s Wort ingredient, hypericin. Apparently, for the
antidepressive action of St. John`s Wort formulations all the plant ingredients
are necessary. For topical administration further investigation is required to
ascertain whether the antioxidative properties remain after having hypericin
isolated in order to find out which of the St. John`s Wort ingredients
explicitely account for the antioxidative properties. The advantages of a
topical application of melissa and sage extract are
the abilities of caffeic acid and ferulic acid to penetrate through the stratum
corneum into the deeper cutaneous layers and provide protection of human
skin there. This was shown as a vehicle pH independent process in vitro
and in vivo by Saija et al.
[49].
Polysaccharides
More
polysaccharides have been tested for their antioxidative properties in the LLA
screening system because of the protective effects of hyaluronic acid (Figure 1C).
In
Figure 4A
the resistant starch novelose 330, a nutrition fibre,
significantly decreased the amount of MDA after UV irradiation. Furthermore,
acacia gum, agar agar, alginic acid, guar gum and xanthan have been tested
without displaying any protective effects.
Figure 4B shows the results of the TBA assay of pectin
and locust bean gum ground by a swing mill for different time intervals.
Whereas pectin showed pro-oxidative effects, the locust bean gum was able to
reduce the amount of lipid peroxidation secondary products after UV radiation
treatment. A correlation between protective behaviour and milling time was not
observed. Antioxidant activity of carob tree (Ceratonia siliqua) plant
material has been shown [52]. Polyphenols were extracted from carob pods and
the in vitro antioxidant activity of the crude polyphenol fraction was
evaluated.
Lipid peroxidation reducing effects for
glycosaminoglycans have been described [53]. The mechanism of these lipid
protecting effects of these polysaccharides seems to be the chelation of
transition metal ions. Sipos and co-workers suggested complexes of the kind L[Fe(OH)3]n where L is the
polysaccharide monomer [54]. This chelation can lead to anti- and pro-oxidative
effects and therefore, has to be tested in every isolated case [55]. Recently
the effects of different metal ions including Zn, Co, Mn etc. on the oxidative
damage and the antioxidant capacity of hyaluronan have been investigated and
compared with iron [56].
Further investigations need to test the compounds
found as protective in this study for their in vivo influences, for
example, on the MED after topical application on subjects. This is of
importance for in vivo evaluation of the generated data and its clinical
interpretation. It is also timely to assess the stability of the antioxidants
in several formulations using the preformulation approach [57] and examine the
penetration patterns of the tested substances [58].
CONCLUSION
Forty seven substances and extracts were
screened for antioxidative effects using an in vitro lipid model system.
For amantadine, bufexamac, tryptophan, melatonin, melanin, propranolol,
diclofenac, ibuprofen and hyaluronic acid, lipid protective effects were
measured using the TBA assay.
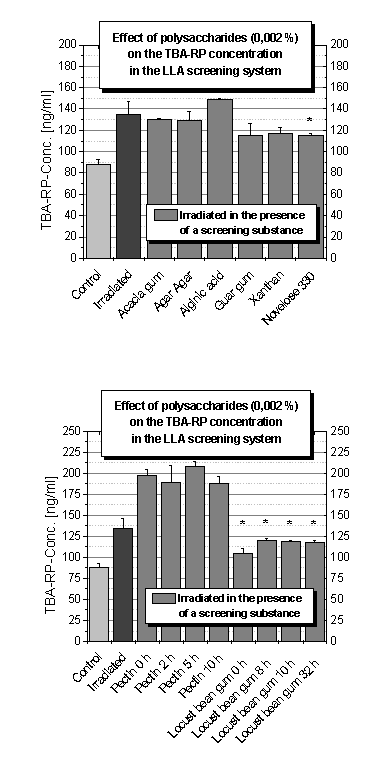
Figure 4. Concentration of thiobarbituric acid reaction products (TBA-RP
Conc.) and influence of ultraviolet irradiation and different test substances
(0.002 % each) in the LLA screening system; A) Influence of polysaccharides; B)
Influence of different pectin and locust bean gum samples resulting from a
swing mill grinding serial.
The flavanoids silibinin and quercetin and
the plant extracts of St. John`s Wort, melissa, sage and buckwheat were able to
reduce the amount of secondary lipid peroxidation products as well. St. John`s
Wort, sage and buckwheat extracts were capable of lowering the TBA assay levels
of the UV irradiated test samples below the value measured for the non-
irradiated control. Among the polysaccharides tested, the resistant starch,
novelose 330 and the different samples of locust bean gum showed antioxidative
properties. On the other hand, pro-oxidative effects were measured for ascorbic
acid, ketoprofen and for the pectin samples.
These results demonstrate a new approach for skin
protection by topical application of substances with antioxidative potency.
Considering human skin as the outermost organ of the
human body and its constant exposure to UV light and oxygen, combined with an
increased iron ion content of the UV exposed skin, topical administration of
some of the substances found in this study, when used in cosmetic and
pharmaceutical semisolid formulations, could be protective for the lipids in
human skin. The use of substances with small molecular weights which are easy to
handle or of molecules naturally occurring in human skin may be of special
advantage.
REFERENCES
[1] Krutmann,
J., New developments in photoprotection of human skin. Skin Pharmacol Appl
Skin Physiol, 14:401‑407, 2001.
[2] Thiele,
J.J., Hsieh, S.N., Briviba, K. and Sies, H., Protein oxidation in human stratum
corneum: susceptibility of keratins to oxidation in vitro and presence of a
keratin oxidation gradient in vivo. J Invest Dermatol, 113:335-339, 1999.
[3] Ogura,
R. and Sugiyama, M., Reactive oxidants in skin: UV induced lipid peroxidation,
in Fuchs J: Packer L (eds), Oxidative stress in
dermatology. 1st ed., Marcel Dekker, INC, New York, NY,
pp 49‑66, 1993.
[4] Samokyszyn,
V.M., Miller, D.M., Reif, D.W. and Aust, S.D. Iron-catalyzed lipid peroxidation,
in Vigo-Pelfrey C (ed), Membrane lipid oxidation, Volume I., 1st ed., CRC
Press, Boca Raton, FL, pp 101‑128, 1990.
[5] Branchaud,
B.P., Free radicals as a result of dioxygen metabolism. Metal Ions Biol Sys,
36:79‑102, 1999.
[6] Fuchs,
J.; Packer, L., Oxidative stress in dermatology, Marcel Dekker, New
York, NY, USA, 1993.
[7] Betteridge,
D.J., What is oxidative stress? Metabolism, 49:3‑8, 2000.
[8] Pincemail,
J., Free radicals and antioxidants in human diseases, in Favier A: Cadet J:
Kalyanaraman B: Fontecave M: Pierre J-L (eds),
Analysis of free radicals in biological systems, 1st ed., Birkhäuser, Basel, pp 83‑98,
1995.
[9] Thiele,
J.J., Oxidative targets in the stratum corneum. A new basis
for antioxidative strategies. Skin Pharmacol Appl Skin Physiol,
14 Suppl 1:87‑91, 2001.
[10] Cunningham,
W.J., Photoaging, in Elsner P: Maibach I (eds),
Cosmeceuticals: Drugs vs. Cosmetics, 1st ed., Marcel Dekker, INC,
New York, NY,
pp 13‑34, 2000.
[11] Sander,
C.S., Chang, H., Salzmann, S., Müller, C.S., Ekanayake-Mudiyanselage, S.,
Elsner, P. and Thiele, J.J., Photoaging is associated with protein oxidation in
human skin in vivo. J Invest Dermatol, 118:618‑625, 2002.
[12] Wertz,
P.W., Lipids and barrier function of the skin. Acta Derm Venereol, Suppl
208:7‑11, 2000.
[13] Shibimato,
T., The role of lipid peroxidation caused by ultraviolet light in skin
diseases. J Toxicol Cut Ocular Toxicol, 13:193‑202, 1994.
[14] Yarosh, D.B., Liposomes in
investigative dermatology. Photodermatol Photoimmunol Photomed,
17:203-212, 2001.
[15] Trommer,
H., Wagner, J., Graener, H. and Neubert, R.H.H., The examination of skin lipid
model systems stressed by ultraviolet irradiation in the presence of transition
metal ions. Eur J Pharm Biopharm, 51:207‑214, 2001.
[16] Trommer,
H., Boettcher, R., Poeppl, A., Hoentsch, J., Wartewig, S. and Neubert, R.H.H.,
Role of ascorbic acid in stratum corneum lipid models exposed to UV
irradiation. Pharm Res, 19:982‑990, 2002.
[17] Trommer,
H., Wartewig, S., Boettcher, R., Poeppl, A., Hoentsch, J., Ozegowski, J.H. and
Neubert, R.H.H., The effects of hyaluronan and its fragments on lipid models
exposed to UV irradiation. Int J Pharm, 254:223‑234, 2003.
[18] Trommer,
H., Plaetzer, M., Raith, K., Podhaisky, H.P., Wohlrab, W. and
Neubert, R.H.H., Examinations of the antioxidative properties of the topically
administered drug bufexamac reveal new insights into its mechanism of action. J
Pharm Pharmacol, 55:1379‑1388, 2003.
[19] Janero,
D.R., Malondialdehyde and thiobarbituric acid-reactivity as diagnostic indices of
lipid peroxidation and peroxidative tissue injury. Free Radic Biol Med,
9:515‑540, 1990.
[20] Rice-Evans, C.A.;
Diplock, A.T.; Symons, M.C.R., Techniques in free radical research, Elsevier,
Amsterdam,
1991.
[21] Cheeseman, K.H., Mechanisms and
effects of lipid peroxidation. Molec Aspects Med, 14:191-197, 1993.
[22] Guy,
R.H.; Hostynek, J.J.; Hinz, R.S.; Lorence, C.R., Metals and the skin. Topical effects and systemic absorption, Marcel Dekker, New York, NY,
1999.
[23] Green,
R., Charlton, R., Seftel, H., Bothwell, T., Mayet, F., Adams,
B., Finch, C. and Layrisse, M., Body iron excretion in man. A
collaborative study. Am J Med,
45:336‑353, 1968.
[24] Buettner,
G.R. and Jurkiewicz, B.A., Catalytic metals, ascorbate and free radicals:
combinations to avoid. Radiation Res, 145:532‑541, 1996.
[25] Kindl, G.; Raab, W., Licht und Haut. Bräunung,
Lichtschutz, Pflege, Govi-Verlag, Eschborn, 1998.
[26] Buege,
J.A. and Aust, S., Microsomal lipid peroxidation. Methods Enzymol,
52:302‑310, 1978.
[27] Podmore,
I.D., Griffiths,
H.R., Herbert, K.E., Mistry, N., Mistry, P. and Lunec, J., Vitamin C exhibits
pro-oxidant properties. Nature, 392:559, 1998.
[28] Chen,
K., Suh, J., Carr, A.C., Morrow, J.D., Zeind, J. and Frei, B., Vitamin C
suppresses oxidative lipid damage in vivo, even in the presence of iron
overload. Am J Physiol Endocrinol Metab,
279:E1406‑E1412, 2000.
[29] Lee,
S.H., Oe, T. and Blair, I.A., Vitamin C-induced decomposition of lipid hydroperoxides
to endogenous genotoxins. Science, 292:2083‑2086, 2001.
[30] Albrecht-Goepfert,
A., Schempp, H. and Elstner, E.F., Modulation of the production of reactive
oxygen species by pre-activated neutrophils by aminoadamantane derivatives. Biochem
Pharmacol, 56:141-152, 1998.
[31] Herraiz,
T., Galisteo, J. and Chamorro, C., L-tryptophan reacts with naturally occurring
and food-occurring phenolic aldehydes to give phenolic
tetrahydro-beta-carboline alkaloids: activity as antioxidants and free radical
scavengers. J Agric Food Chem, 51:2168‑2173, 2003.
[32] Baskin,
S.I.; Salem, H., Oxidants, Antioxidants, and Free Radicals, 1st ed. Taylor
& Francis, Washington, DC, USA, 1997.
[33] Kalka,
K., Mukhtar, H., Turowski-Wanke, A. and Merk, H., Biomelanin antioxidants in
cosmetics: assessment based on inhibition of lipid peroxidation. Skin
Pharmacol Appl Skin Physiol, 13:143‑149, 2000.
[34] Aruoma,
O.I., Smith, C., Cecchini, R., Evans, P.J. and Halliwell, B., Free radical
scavenging and inhibition of lipid peroxidation by beta-blockers and by agents
that interfere with calcium metabolism. A
physiologically-significant process? Biochem Pharmacol,
42:735-743, 1991.
[35] Zang,
L.Y., Cosma, G., Gardner,
H. and Vallyathan, V., Scavenging of reactive oxygen species by melatonin. Biochim
Biophys Acta, 1425:469‑477, 1998.
[36] Fischer,
T.W. and Elsner, P., The antioxidative potential of melatonin in the skin. Curr
Probl Dermatol, 29:165‑174, 2001.
[37] Dreher,
F., Gabard, B., Schwindt, D.A. and Maibach, H.I., Topical melatonin in
combination with vitamins E and C protects skin from ultraviolet-induced
erythema: a human study in vivo. Br J Dermatol, 139:332‑339, 1998.
[38] Mouithys-Mickalad,
A.M, Zheng, S.X., Deby-Dupont, G.P., Deby, C.M., Lamy, M.M., Reginster, J.Y.
and Henrotin, Y.E., In vitro study of the antioxidant properties of non
steroidal anti-inflammatory drugs by chemiluminescence and electron spin
resonance (ESR). Free Radic Res, 33:607‑621, 2000.
[39] Hamburger,
S.A.
and McCay, P.B., Spin trapping of ibuprofen radicals: evidence that ibuprofen
is a hydroxyl radical scavenger. Free Radic Res Commun, 9:337‑342,
1990.
[40] Kreussler Pharma, Gabrilenâ Gel Gebrauchsinformation, Chemische Fabrik Kreussler & Co GmbH.,
Wiesbaden, 1998.
[41] Radschuweit,
A., Rüttinger, H.H., Nuhn, P., Wohlrab, W. and Huschka, C., UV-induces
formation of hydrogen peroxide based on the photochemistry of ketoprofen. Photochem
Photobiol, 73:119‑127, 2001.
[42] Rice-Evans, C.A.;
Packer, L., Flavonoids in health and disease, Marcel Dekker, INC,
New York, NY,
USA, 2003.
[43] Boehm, H., Boeing, H., Hempel, J., Raab, B.
and Kroke, A., Flavonole, Flavone und Anthocyane als natürliche Antioxidantien
der Nahrung und ihre mögliche Rolle bei der Prävention chronischer
Erkrankungen. Z
Ernährungswiss, 37:147‑163,
1998.
[44] Kessler,
M., Ubeaud, G. and Jung, L., Anti- and pro-oxidant activity of rutin and
quercetin derivatives. J Pharm Pharmacol, 55:131‑142, 2003.
[45] Dehmlow,
C., Murawski, N. and de Groot, H., Scavenging of reactive oxygen species and
inhibition of arachidonic acid metabolism by silibinin in human cells. Life
Sci, 58:1591‑1600, 1996.
[46] Mira,
L., Silva, M. and Manso, C.F., Scavenging of reactive oxygen species by
silibinin dihemisuccinate. Biochem Pharmacol, 48:753‑759, 1994.
[47] Valentao,
P., Fernandes, E., Carvalho, F., Andrade, P.B., Seabra, R.M. and de Lourdes
Bastos, M., Antioxidant activity of Hypericum androsaemum infusion: scavenging
activity against superoxide radical, hydroxyl radical and hypochlorous acid. Biol
Pharm Bull, 25:1320-1323, 2002.
[48] Hohmann,
J., Zupko, I., Redei, D., Csanyi, M., Falkay, G., Mathe, I. and Janicsak, G.,
Protective effects of the aerial parts of Salvia officinalis, Melissa
Officinalis and Lavandula angustifolia and their constituents against
enzyme-dependent and enzyme-independent lipid peroxidation. Planta Med,
65:576‑578, 1999.
[49] Saija,
A., Tomaino, A., Trombetta, D., De Pasquale, A., Uccella, N., Barbuzzi, T.,
Paolino, D. and Bonina, F., In vitro and in vivo
evaluation of caffeic and ferulic acids as topical photoprotective agents. Int
J Pharm, 199:39‑47, 2000.
[50] Srinivasan,
A., Menon, V.P., Periaswamy, V. and Rajasekaran, K.N., Protection of pancreatic
beta-cell by the potential antioxidant bis-o-hydroxycinnamoyl methane, analogue
of natural curcuminoid in experimental diabetes. J Pharm Pharm Sci,
6:327‑333, 2003.
[51] Mukoda,
T., Sun, B. and Ishiguro, A., Antioxidant activities of buckwheat hull extract
toward various oxidative stress in vitro and in vivo. Biol Pharm Bull,
24:209‑213, 2001.
[52] Kumazawa,
S., Taniguchi, M., Suzuki, Y., Shimura, M., Kwon, M.S. and Nakayama, T.,
Antioxidant activity of polyphenols in carob pods. J Agric Food Chem,
50:373‑377, 2002.
[53] Albertini,
R., Passi, A., Abuja,
P.M. and De Luca, G., The effect of glycosaminoglycans on lipid peroxidation.
Int J Mol Med, 6:129‑136, 2000.
[54] Sipos,
P., Berkesi, O., Tombacz, E., St
Pierre, T.G. and Webb, J., Formation of spherical iron
(III) oxyhydroxide nanoparticles sterically stabilized by chitosan in aqueous
solutions. J Inorg Biochem, 95:55‑63, 2003.
[55] Gutteridge,
J.M., Richmond,
R. and Halliwell, B., Inhibition of the iron-catalysed formation of hydroxyl
radicals from superoxide and of lipid peroxidation by desferrioxamine. Biochem J, 184:469‑472,
1979.
[56] Balogh,
G.T., Illes, J., Szekely, Z., Forrai, E. and Gere, A., Effect of different metal
ions on the oxidative damage and antioxidant capacity of hyaluronic acid. Arch Biochem Biophys, 410:76‑82, 2003.
[57] Proniuk,
S., Liederer, B.M. and Blanchard, J., Preformulation study of epigallocatechin
gallate, a promising antioxidant for topical skin cancer prevention. J Pharm
Sci, 91:111‑116, 2002.
[58] Lademann,
J., Weigmann, H., Rickmeyer, C., Barthelmes, H., Schaefer, H., Mueller, G. and
Sterry, W., Penetration of titanium dioxide microparticles in a sunscreen
formulation into the horny layer and the follicular orifice. Skin Pharmacol
Appl Skin Physiol, 12:247‑256, 1999.
JPPS Contents
Published by the Canadian Society for
Pharmaceutical Sciences.
Copyright © 1998 by the Canadian
Society for Pharmaceutical Sciences.
http://www.cspscanada.org/
CSPS Home |
JPPS Home |
Search |
Subscribe to JPPS