J Pharm Pharmaceut Sci (www.cspscanada.org) 8(3):394-399, 2005
Safranal, a constituent of
Crocus sativus
(saffron), attenuated cerebral ischemia induced oxidative damage in rat
hippocampus
Hossein Hosseinzadeh1, Hamid R. Sadeghnia2
1-
2-
Department of Pharmacology, Faculty of
Medicine,
Received March 19, 2005; Revised June 15, 2005, Accepted August 15, 2005, Published August 22, 2005
Corresponding author:
Hossein Hosseinzadeh,
Abstract Increased
oxidative stress has been implicated in the mechanisms
of delayed neuronal cell death following cerebral ischemic insult. In this
study, we investigated whether safranal, an active constituent of Crocus sativus
L. stigmas, may ameliorate ischemia-reperfusion injury (IRI)-induced oxidative
damage in rat hippocampus. Male NMRI rats were divided
into six groups, namely, sham, control, ischemia and ischemia treated with
safranal (four groups). The transient global cerebral ischemia was induced using
four-vessel-occlusion method for 20 min. Safranal was
injected intraperitoneally (727.5 mg/kg, 363.75 mg/kg, 145.5 mg/kg, and
72.75 mg/kg body weight) 5 min. prior to reperfusion and the administration was
continued every 24 hours for 72 hours after induction of ischemia. The markers
of oxidative stress including thiobarbituric acid reactive substances (TBARS),
total sulfhydryl
(SH) groups and antioxidant capacity of hippocampus (using FRAP assay) were
measured. The transient global cerebral ischemia induced a significant increase in TBARS
levels (p<0.001), decrement in both antioxidant power (FRAP value)
(p<0.05) and total sulfhydryl (SH) concentrations (p<0.001) in
comparison with sham-operated animals. Following safranal administration the total
SH contents (3.2 vs. 0.7 µmol/g, p<0.001, safranal 727.5 mg/kg) and
antioxidant capacity (4.12 vs. 1.16 µmol/g, p<0.001; 727.5 mg/kg) were
elevated in hippocampus in comparison with ischemic group. The MDA level was declined
significantly in hippocampus (52.31 vs. 159.70 nmol/g, p<0.001; 727.5
mg/kg). It is concluded that safranal have some protective effects
on different markers of oxidative damage in hippocampal tissue from
ischemic rats.
Introduction
As the brain’s high oxygen
consumption, high lipid contents, especially polyunsaturated fatty acids, high
concentrations of transition metals, and low antioxidants activity, the
oxidative stress has been implicated as a potential contributor to the
pathogenesis of acute central nervous system (CNS) injury. After brain injury,
for example by ischemia, the production of reactive oxygen species (ROS) may
increase, leading to tissue damage via several different cellular molecular
pathways. Radicals can cause damage to cardinal cellular components such as
lipids, proteins, and nucleic acids (e.g., DNA), leading to subsequent cell
death by modes of necrosis or apoptosis. Therefore, the use of antioxidants,
free radical scavengers or trapping agents may be rational in acute and chronic
CNS injuries, especially cerebral ischemia-reperfusion injury (IRI) [1-3].
Recently, there is overwhelming attention to plant products and natural agents
that can limit free radical-mediated injuries, for better therapeutic
management of IRI.
Crocus sativus L., commonly known as saffron, is used in folk medicine as an antispasmodic, eupeptic,
gingival sedative, anticatarrhal, nerve sedative, carminative, diaphoteric,
expectorant, stimulant, stomachic, aphrodisiac and emmenagogue [4].
Furthermore, modern pharmacological studies have demonstrated that saffron
extract or its active constituents have anticonvulsant [5], antidepressant [6],
anti-inflammatory [7] and antitumour effects, radical scavenger as well as
learning and memory improving properties [4, 8-11] and promote the diffusivity
of oxygen in different tissues [4]. Saffron extract also has chemopreventive
and genoprotective effects and protects from genotoxins-induced oxidative
stress in mice [12-15].
The aim of present
study was to assess the protective effects of safranal, the active constituent
of Crocus sativus L. stigmas, on ischemia-reperfusion
injury (IRI)-induced oxidative damage in rat hippocampus.
Materials and Methods
Animals
Adult male NMRI rats weighing
200-300 g were used throughout the study. All of them
were kept in the same room under a constant temperature (22 ± 2 °C) and illuminated 7:00 a.m. to 7:00
p.m., with food pellets and water available ad libitum.
The animals were divided into six groups, each of which contained 8
rats. Group 1 was the sham group in which only surgery was
done without induction of ischemia; group 2 was the control group in
which saline solution was given intraperitoneally. In groups 3-6, safranal (727.5
mg/kg, 363.75 mg/kg, 145.5 mg/kg and 72.75 mg/kg, i.p., [6]) was administrated 5
min prior to reperfusion and the administration was continued
every 24 hours for 72 hours after the induction of ischemia.
Chemicals
DTNB (2, 2´-dinitro-5,
5´-dithiodibenzoic acid), TPTZ (2, 4, 6-tri (2-pyridyl)-1, 3, 5-triazine), TBA
(2-thiobarbituric acid), n-butanol, tris, Na2EDTA, sodium acetate,
glacial acetic acid, phosphoric acid, potassium chloride, tetramethoxypropane
(TMP), ferric chloride (FeCl3.6H2O), ferrous sulfate and
hydrochloric acid was obtained from Merck. Safranal was
purchased from Fluka.
Induction of global cerebral ischemia
Global cerebral ischemia was
induced using the four-vessel occlusion method (4-VO), described by Pulsinelli et
al [16]. Briefly, under intraperitoneal ketamine/xylazine anesthesia
(60 mg/kg and 6 mg/kg, respectively), the alar foramina of first cervical
vertebrae were exposed and the vertebral arteries were electrocauterized
permanently. On the next day and under brief anesthesia, the common carotid
arteries (CCAs) were dissected from surrounding
tissues and temporarily ligated using the microvascular clamps for 20 min. At the end of the ischemic
period, the ligature was removed and reperfusion was
supplied. After maintaining of animals in suitable situation for 72 hours, the
animals were decapitated and the hippocampus portion
was homogenized in 1.5% cold KCl solution to give a 10% homogeny suspension and
used for biochemical assays.
Tiobarbituric acid reactive species (TBARS) measurement
The lipid peroxidation level of
the hippocampus portion was measured as malondialdehyde (MDA) which is the end
product of lipid peroxidation, and reacts with thiobarbituric acid (TBA) as a
thiobarbituric acid reactive substance (TBARS) to produce a red colored complex
which has peak absorbance at 532 nm [17].
3 ml phosphoric
acid (1%) and 1 ml TBA (0.6%) was added to 0.5 ml of
homogenate in a centrifuge tube and the mixture was heated for 45 min in a
boiling water bath. After cooling, 4 ml of n-butanol was added to the mixture
and vortex-mixed for 1 min followed by centrifugation at 20000 rpm for 20 min.
The organic layer was transferred to a fresh tube and
its absorbance was measured at 532 nm. The standard curve of MDA was constructed over the concentration range of 0-40 µM
[18].
Ferric Reducing / Antioxidant Power (FRAP) assay
The FRAP assay measures the
change in absorbance at 593 nm owing to the formation of a blue colored FeII-tripyridyltriazine
compound from the colorless oxidized FeIII form by the action of
electron donating antioxidants [19].
The FRAP reagent
consist of 300 mM acetate buffer (3.1 g sodium acetate + 16 ml glacial acetic
acid, made up to 1 liter with distilled water; pH=3.6), 10 mM TPTZ in 40 mM HCl
and 20 mM FeCl3.6H2O in the ratio of 10:1:1.
Briefly, 50 μl
of homogenate was added to 1.5 ml freshly prepared and prewarmed (37 şC) FRAP
reagent in a test tube and incubated at 37 şC for 10 min. The absorbance of the
blue colored complex was read against reagent blank (1.5 ml FRAP reagent + 50 μl
distilled water) at 593 nm. Standard solutions of Fe II in the range
of 100 to 1000 mM were prepared from ferrous sulphate (FeSO4.7H2O)
in distilled water. The data was expressed as mmol
ferric ions reduced to ferrous form per liter (FRAP value) [20].
Total sulfhydryl (SH) groups assay
Total
SH groups were measured using DTNB (2, 2´-dinitro-5,
5´-dithiodibenzoic acid) as the reagent. This reagent reacts with the SH groups
to produce a yellow colored complex which has peak absorbance at 412 nm [21].
Briefly, 1 ml Tris-EDTA buffer (pH=8.6)
was added to 50 μl homogenate in 2 ml cuvettes and sample absorbance was
read at 412 nm against Tris-EDTA buffer alone (A1). Then 20 μl DTNB reagent (10 mM in methanol) was added to the
mixture and after 15 min (stored in laboratory temperature), the sample
absorbance was read again (A2). The absorbance of DTNB reagent was also read as a blank (B). Total thiol concentration (mM)
was calculated from the following equation:
Total thiol concentration (mM) = (A2-A1-B)
× 1.07/0.05 × 13.6
Statistical analysis
Data are
expressed as mean ± SEM. Statistical analysis was performed using
one-way ANOVA followed by Tukey-Kramer post-hoc test for multiple
comparisons. The p-values less than 0.05 were considered
statistically significant.
Results
Change of MDA levels by safranal
The degree of free radical
damage following IRI was assessed using lipid peroxidation, which was measured as MDA levels. There was an increase (64.2 %)
in the MDA levels following IRI as compared with sham-operated animals (159.70
± 15.90 vs. 97.25 ± 5.18 nmol/g tissue, p<0.001) (Figure 1). Safranal pretreatment resulted in a significant and
dose-dependently reduction in the free radical-mediated lipid peroxidation as
indicated by a decrease in the MDA levels, at various dose levels. In
safranal-pretreated groups with doses 145.5 mg/kg, 363.75 mg/kg and 727.5
mg/kg, TBARS levels were 52.31, 76.85 and 98.74 nmol/g tissue, respectively
(Figure 1).
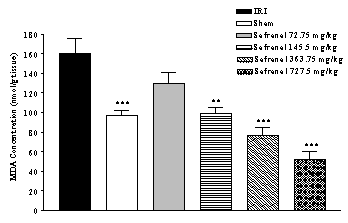
Figure 1: Effect of safranal on lipid peroxidation following global cerebral ischemia. MDA levels were measured in 10% homogenates of hippocampus portion from rats subjected to 20 min of ischemia. All drugs were administrated intraperitonealy 5 min prior to reperfusion. Values are mean±SEM (n=8). ***p<0.001 as compared with vehicle (normal saline) treated animals (One-way ANOVA followed by Tukey-Kramer test)
Change of FRAP value by safranal
IRI caused a significant
reduction in FRAP value (53.2 %) of homogenate samples as compared with
sham-operated animals (1.16 ± 0.2 vs. 2.48 ± 0.16 µmol/g tissue, p<0.001)
(Figure 2). Safranal pretreatment increased antioxidant power (FRAP value) of
brain homogenate samples, in non-dose dependent manner (from 1.16 ± 0.2 to 4.12
± 0.33 µmol/g tissue, p<0.001; 727.5 mg/kg) (Figure 2).
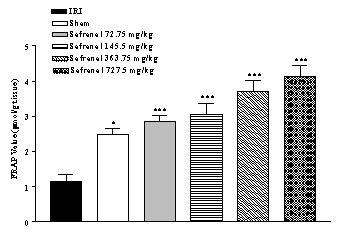
Figure 2:
Effect of safranal on
antioxidant power of hippocampus homogenate samples following global cerebral
ischemia. FRAP values were measured in 10% homogenate samples from rats
subjected to 20 min of ischemia. All drugs were administrated intraperitonealy
5 min prior toreperfusion. Values are mean ± SEM (n=8). *p<0.05,
***p<0.001 as compared with vehicle (normal saline) treated animals (One-way
ANOVA followed by Tukey-Kramer test)
Effect of safranal on total thiol concentration
Following ischemia-reperfusion injury a significant reduction (77.6 %) in total SH groups (0.710 ± 0.068 vs. 3.170 ± 0.140 µmol/g tissue, p<0.001) in homogenate samples were observed (Figure 3). Safranal pretreatment induced a significant and dose dependently elevation in total thiol concentration, as compared with control group (from 0.710 ± 0.068 to 3.180 ± 0.075 µmol/g tissue, p<0.001; 727.5 mg/kg) (Figure 3).
Discussion
A great deal of effort has been directed toward searching for new compounds that
can be used for protection of cerebral ischemia–reperfusion injury. The results
obtained in the present investigation suggest that safranal, one of the
constituents of saffron stigmas with monoterpenoid structure, has an overall
protective effect against cerebral ischemia-reperfusion injury-induced
oxidative stress in a rat model.
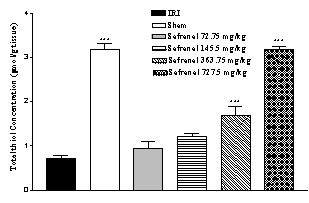
Figure 3: Effect of safranal on total thiol concentrations following global
cerebral ischemia. Total sulfhydryl (SH) groups were measured
in 10% hippocampus homogenate samples from rats subjected to 20 min of
ischemia. All drugs were administrated intraperitonealy 5 min prior to
reperfusion. Values are mean ± SEM (n=8). ***p<0.001 as compared with
vehicle (normal saline) treated animals (One-way ANOVA followed by Tukey-Kramer
test)
A number of
processes have been implicated in the pathogenesis of
oxygen deprivation–induced cell injury. These include the disturbances of cell
calcium homeostasis, depletion of adenine nucleotides, activation of enzymes
like phospholipases with production of toxic lipid metabolites, proteases and
endonucleases and generation of free radicals (ROS) that can cause oxidative
damage to cellular macromolecules [22]. It is well documented that oxidative
stress is a major common pathway of cellular injury following neurological and
neurodegenerative disorders such as ischemia-reperfusion, seizure, Parkinson
and Alzheimer’s disease and antioxidant therapy have been well documented to
protect against CNS injuries [3, 23, 24].
The large numbers
of polyunsaturated fatty acids (PUFAs) make cell membranes particularly
vulnerable to lipid peroxidation. The oxidation of PUFAs causes them to be more
hydrophilic, thereby altering the structure of the membrane with resultant
changes in fluidity and permeability. Lipid peroxidation can also inhibit the
function of membrane bound receptors and enzymes [22, 23].
We assessed the effect of
safranal on lipid peroxidation, which was measured in
terms of MDA, a stable metabolite of the free radical-mediated lipid
peroxidation cascade. The MDA levels increased significantly (p<0.001)
following cerebral IRI. Safranal reversed the increase of MDA levels to a
considerable extent, thereby confirming its antioxidant role in IRI.
Sulfhydryl (SH)
groups are highly-reactive constituents of protein
molecules, and they participate in important biochemical and metabolic process
such as cell division, blood coagulation, maintance of protein systems and
enzymatic activation including antioxidant enzymes (catalase, superoxide
dismutase, etc.) [25]. There are also important scavengers of oxygen-derived
free radicals [26]. SH groups known to be sensitive to
oxidative damage and depleted following ischemic insult [27], therefore we studied the effect of this agent on
the total thiol concentration during IRI. Similarly, in our studies, total
sulfhydryl groups were decreased following
ischemic-reperfusion injury. Safranal pretreated rats exhibited higher SH
contents than their respective controls in the dose related pattern, indicating
that safranal helped in replenishing the total thiol pool.
Under acute and chronic
pathologic conditions such as ischemia, the balance between oxidant and
antioxidant systems has been interrupted [2, 3, 28]. Therefore, we evaluate the antioxidant or reducing potential of
hippocampus homogenate samples following IRI, using FRAP assay. As expected
following IRI, a significant reduction in antioxidant power, as indicated by
FRAP value, was observed. Safranal increased the
antioxidant power of homogenate samples of hippocampus.
Saffron has
chemopreventive effects and its extract inhibits tumor growth in vivo and in
vitro [12, 13, 29-34]. Escribano et al showed that saffron extract and
its constituents; crocin, safranal and picrocrocin inhibit the growth of human
cancer cells (Hella cells) in vitro [9]. Abdullaev and Frenkel also showed
saffron affect intracellular nucleic acid and protein synthesis [35, 36].
Another study (El Daly) demonstrated protective effects of saffron extract
against cisplatin induced toxicity in rats [37]. Saffron extract also has
radical scavenger properties [4] and protects from genotoxicity as well as
genotoxins-induced oxidative stress in mice [14, 15]. Premkumar et al showed
oral pretreatment with the saffron aqueous extract (40 and 80 mg/kg) for five
consecutive days inhibit genotoxins-induced oxidative stress in mice liver. In
this study, an increase in the levels of glutathione (GSH) concentration as
well as the activities of glutathione S-transferase (GST), glutathione
peroxidase (GPx), catalase and superoxide dismutase (SOD) were observed,
however, normal levels of GSH could not be attained [15].
Among the
constituent of saffron stigmas, crocins and crocetin derivatives are most
abundant with established antioxidant and antitumor effects [4, 8, 13]. These carotenoids scavenge free radicals, especially
superoxide anions and thereby may protect cells from oxidative stress [38]. In
rats, crocin dyes are known to exert protective
effects against acute hepatic damage induced by aflatoxin B1 and
dimethylnitrosamine [39]. It has been shown that
crocetin, the deglycosylated crocin derivative, has protective effects on
aflatoxin B1-induced hepatotoxicity and protects rat primary
hepatocytes against oxidative damage [40-42]. Cancer chemopreventive as well as
antitumor activities were also reported for crocins
and crocetin derivatives in different assay systems [9, 43-46].
There
are several reports about the antioxidant activity and anti-inflammatory
effects of some monoterpenoids such as a-pinene.
Moreover, there have been shown monoterpenoids such as terpineol and linalool
have depressant effects on central nervous system, in vivo [47] and linalool
competitively inhibits glutamate receptors [48]. There are no reports about
clinical uses of safranal. Much more basic pharmacological
and toxicological studies need for clinical trials to evaluate the safety, tolerability
and efficacy of safranal.
Our previous studies showed that safranal has a potent depressant effect on
CNS and clearly suppress pentylenetetrazole-induced seizures (unpublished data)
[49]. Its may be concluded that protective effect of safranal on
ischemia-reperfusion injury, at least partly, due to these mechanisms, but it
needs to be further investigated.
It is concluded that safranal have some protective effects on different markers of oxidative damage in hippocampal tissue from ischemic rats.
Acknowledgements
The authors are thankful to the Research Council, Mashhad University of Medical Sciences for financial support.
References
[1]
White BC, Sullivan JM, DeGracia DJ, O’Neil BJ, Neumar RW, Grossman LI, Rafols
JA, Krause GS. Brain ischemia and reperfusion: molecular
mechanisms of neuronal injury. J Neuro Sci 2000, 179: 1-33.
[2] Streck EL, Vieira PS, Wannmacher CMD, Dutra-Filho CS,
Wajner M, Wyse ATS. In vitro effect of homocysteine on some
parameters of oxidative stress in rat hippocampus. Metab Brain Disease
2003, 18: 147-54.
[3]
Gilgun-Sherki Y, Rosenbaum Z, Melamed E, Offen D. Antioxidant Therapy in Acute
Central Nervous System Injury: Current State. Pharm Rev 2002, 54:271-84.
[4] Rios jl, Recio MC, Ginger RM, Manz S. An update
review of saffron and its active constituents. Phytother Res 1996, 10:
189-93.
[5]
Hosseinzadeh H, Khosravan V. Anticonvulsant effects of aqueous and ethanolic
extracts of Crocus sativus L. stigmas in mice. Arch Irn Med 2002,
5: 44-7.
[6] Hosseinzadeh H, Karimi Gh,
Niapoor M. Antidepressant effects of Crocus sativus stigma extracts and
its constituents, crocin and safranal, in mice. Acta Hort (ISHS) 2004, 650:
435-45.
[7] Hosseinzadeh H, Younesi HM.
Antinociceptive and anti-inflammatory effects of Crocus sativus L.
stigma and petal extracts in mice. BMC Pharmacol 2002, 2: 1-8.
[8]
Abdullaev FI. Biological effects of saffron. Biofactors
1993, 4: 83-6.
[9]
Escribano J, Alonso GL, Coca-Prados M, Fernandez JA. Crocin, safranal and
picrocrocin from saffron (Crocus sativus L.) inhibit the growth of human
cancer cells in vitro. Cancer Lett 1996, 100: 23-30.
[10] Zhang
YX, Sugiura M, Saito H, Shoyama Y. Acute effects of Crocus sativus L. on
passive avoidance performance in mice. Biol Pharmacol Bull 1994, 17: 217-21.
[11]
Abe K, Sugiura M, Ymaguchi S, Shoyama Y, Saito H. Safrron extract prevents
acetaldehyde-induced inhibition of long-term potentiation in the rat dentate
gyrus in vivo. Brain Res 1999, 851: 287-89.
[12]
Abdullaev FI, Caballero-Ortega H, Riveron-Nigrete L, Pereda-miranda R,
Rivera-Luna R, Manuel Hernandez J, Perez-Lopez I, Espinosa-Aguirre JJ. In vitro evaluation of chemopreventive potential of saffron.
Rev Inves Clin 2002, 54: 430-36.
[13] Nair SC,
[14]
Premkumar K, Abraham SK, Santhiya ST, Gopinath PM, Ramesh A. Inhibition of
genotoxicity by saffron (Crocus sativus L.) in mice. Drug Chem Toxicol
2001, 24: 421-28.
[15]
Premkumar K, Abraham SK, Santhiya ST, Ramesh A. Protective effects of saffron (Crocus
sativus L.) on genotoxins-induced oxidative stress in swiss albino mice.
Phytother Res 2003, 17: 614-17.
[16]
Pulsinelli WA, Brierley JB. A new model of bilateral
hemispheric ischemia in the unanesthetized rat. Stroke 1979, 10: 268-72.
[17]
Fernandez J, Perez-Alvarez JA, Fernandez-lopez JA.
Thiobarbituric acid test for monitoring lipid
oxidation in meat. Food Chem 1997, 99: 345-53.
[18] Uchiama M,
Miahara M. Determination of malonaldehyde precursor in tissues by
thiobarbituric acid test.
Anal Biochem 1978, 86: 279-86.
[19]
Benzie IFF, Strain J. The ferric reducing ability of plasma (FRAP) as a measure
of antioxidant power: The FRAP assay. Anal Biochem 1996, 239: 70-6.
[20]
Benzie IFF, Strain JJ. Ferric reducing/antioxidant power assay: direct measure
of total antioxidant activity of biological fluids and modified version for
simultaneous measurement of total antioxidant power and ascorbic acid
concentration. Methods Enzymol 1999, 299: 15-27.
[21] Ellman G. Tissue sulfhydryl groups. Arch Biochem Biophys 1959, 82:
70-7.
[22]
Fisher M. Stroke therapy.
2nd ed. Butter worth-Heinemann 2001, 25-50.
[23]
Love S, Oxidative stress in brain ischemia. Brain Pathol 1999, 9: 119-31.
[24]
Sun AY, Chen YM. Oxidative stress and neurodegenerative
disorders. J Biomed Sci 1998, 401-414.
[25]
Jansen EV. Sulfhydryl-disulfide interchanges. Science 1959, 130: 1319-1323.
[26]
Dormandy TL. An approach to free radicals in medicine and
biology. Acta Physiol Scand 1980 492, 153-168.
[27]
Soszynski M, Bartosz G. Decrease in accessible thiols as an index of oxidative
damage to membrane proteins. Free Rad Biol Med 1997, 23: 463–69.
[28] Abdollahi M, Ranjbar R, Shadnia S,
Nikfar S, Rezaie A. Pesticides and oxidative stress: a review. Med Sci Monit
2004, 10: 141-47.
[29]
Salomi MJ, Nair SC, Panikkar KR. Inhibitory effects of Nigella sativa and
Crocus sativus on chemical carcinogenesis in mice and its non-mutagenic
activity. Proc Ker Sci Congress 1990, 3: 125.
[30] Salomi MJ,
[31]
Abdullaev FI. Cancer chemopreventive and tumoricidal
properties of saffron (Crocus sativus). Exptl Biol Med 2002, 227:
20-25.
[32]
Das I, Chakrabarty RN, Das S. Saffron can prevent chemically induced skin
carcinogenesis in swiss albino mice. Asian Pac J Cancer Prev 2004, 4: 70-76.
[33]
Nair SC, Pannikar B, Pannikar KR. Antitumor activity of saffron (Crocus
sativus). Cancer Lett 1991, 57: 109-114.
[34] Nair SC, Varghese CD, Pannikar KR,
[35]
Abdullaev FI, Frenkel GD. The effect of saffron on
intracellular DNA, RNA and protein synthesis in malignant and non-malignant
human cells. Biofactors 1992, 4: 43-45.
[36]
Abdullaev FI, Frenkel GD. Effect of saffron on cell colony formation and
cellular nucleic acid and protein synthesis. Biofactors 1992, 3: 201-204.
[37] El Daly ES. Protective effect of cysteine
and vitamin E, Crocus sativus and Nigella sativa extracts on
cisplatin-induced toxicity in rats. J Pharm Belg 1998, 53:87-95.
[38] Bors W, Saran M, Michel C. Radical
intermediates involved in the bleaching of the carotenoid crocin. Hydroxyl radicals, superoxide anions and hydrated electrons.
Int J Radiat Biol Relat Stud Phys Chem Med 1982, 41: 493-501.
[39] Lin JK, Wang CJ. Protection of crocin
dyes on the acute hepatic damage induced by aflatoxin B1 and
dimethylnitrosamine in rats. Carcinogenesis 1986, 7:595-599.
[40] Wang CJ, Shiow SJ, Lin JK. Effects of crocrtin on the heptotoxicity and hepatic DNA binding of
aflatoxin B1 in rats. Carcinogenesis 1991, 12: 459-462.
[41] Wang CJ, Hsu JD, Lin JK. Suppresion of aflatoxin B1-induced hepatotoxicity lesions by
creocetin. Carcinogenesis 1991, 12:1807-1810.
[42] Tseng TH,
[43] Garcia-Olmo DC, Riese HH, Escribano
J, Ontanon J, Fernandez JA, Atienzar M, Garcia-Olmo D. Effects of long term
treatment of colon adenocarcinoma with crocin, a caretinoid from saffron (Crocus
sativus): an experimental study in the rat. Nutr Cancer 1999, 35: 120-126.
[44] Tarantilis PA, Morjani H, Polissou M,
Manafait M. Inhibition of growth and induction of differentiation of
promyelocytic leukemia (HL-60) by caretinoids from Crocus sativus L.
AntiCancer Res 1994, 14: 1913-1918.
[45] Konoshima T, Takasaki M, Tokuda H,
Morimoto S, Tanaka H, Kawata E, Xuan LJ, Saito H, Sugiura M, Molnar J, Shoyama
Y. Crocin and crocetin derivatives inhibit skin tumor promotion in mice.
Phytother Res 1998, 12: 400-404.
[46] Wang CJ, Lee MJ, Chang MC, Lin JK.
Inhibition of tumor promotion in benzo(a)pyrene-initaited
CD-1 mouse skin by crocetin. Carcinogenesis 1995, 16: 501-506.
[47] Perry NSL, Bollen C, Perry
EK, Ballard C. Salvia for
dementia therapy: review of pharmacological activity and pilot tolerability
clinical trial. Pharmacol Biochem Behav 2003, 75: 651-659
[48]
Silva Brum LF, Elisabetsky E, Souza D. Effect of linalool on [3H]
MK801 and [3H] muscimol binding in mouse cortical membranes.
Phytother Res 2001, 15: 422–25.
[49] Hosseinzadeh H, Talebzadeh F. Anticonvulsant evaluation of safranal and crocin from Crocus sativus in mice. Fitoterapia. 2005, In Press.
Published by the Canadian Society for Pharmaceutical Sciences.
Copyright © 1998 by the Canadian Society for Pharmaceutical Sciences.
CSPS Home | JPPS Home | Search | Subscribe to JPPS