J Pharm Pharmaceut Sci (www.cspscanada.org) 8(3):528-535, 2005
Isolation and characterization of methyl esters and derivatives from Euphorbia kansui (Euphorbiaceae) and their inhibitory effects on the human SGC-7901 cells.
Fa-Rong Yu1, Xiu-Zhen Lian1, Hong-Yun Guo2, Peter M. McGuire3, Ren-De Li4, Rui Wang4, Fa-Hong Yu3
1 School of Public Security, Gansu Institute of Political Science
and Law, Lanzhou 730070, China
2 Department of pharmacology, Gansu Academy of Medical Sciences, Lanzhou 730050,
China
3 Department of Biochemistry and Molecular Biology,
University of Florida,
Gainesville, FL 32610, USA
4 School of Life Sciences,
Lanzhou University, Lanzhou 730000, China
Received May 16, 2005, Revised September 14, 2005, Accepted September 19, 2005, Published September 27, 2005
Corresponding
Authors: Rui Wang,
Abstract PURPOSE. In this study, the inhibitory activity of the methyl
esters and derivatives extracted from Euphorbia kansui (Euphorbiaceae)
and their effect on apoptosis and cell cycle distribution in the human gastric
cancer cell line (SGC-7901) were evaluated. METHODS. The inhibitory
activity of the methyl esters and derivatives was evaluated by using trypan-blue,
MTT (3-(4, 5-dimethyl thiazol-2yl) - 2, 5-diphenyltetrazolium bromide), and FCM (flow
cytometry) assays. 5-fluorouracile
(5-FU) was used for a positive control. RESULTS. Six new methyl esters
and derivatives were extracted from the root of E. kansui. Subjecting
the SGC-7901 cell line to the extract indicated that methyl ester derivatives
could initiate growth inhibition and induce apoptosis in these tumor cells. The
inhibitory rates as measured from trypan-blue and MTT assays were significantly
increased and are comparable to those of the common antitumor agent 5-FU. In
addition, the methyl ester extract effectively inhibited the proliferation of
SGC-7901 cells by interfering with the progression of the cells through the G1
phase of the cell cycle. CONCLUSION. The current study indicates that methyl
esters might be a promising chemopreventive and chemotherapeutic agent for
treating various forms of cancer by causing apoptosis and proliferation
inhibition.
INTRODUCTION
Euphorbia kansui (Euphorbiaceae), commonly known as Mao Eryan
in Chinese medicine grows widely in northwestern and northern China. E.
kansui has been characterized as one of the best therapeutically among
those Euphorbia species that have been used in herbal remedies for use
as an analgesia (1) and for treating
ascites, leukemia (2, 3), whooping cough, pancreatitis, and some tumors
(4, 5).
The chemical
constituents of E. kansui and their biological effects, such as antiinflammatory,
antitumor, immunomodulatory, and antiproliferative activities, have been reported
in recent research (5-9). Besides polycyclic diterpenes, ingenane-type
diterpenes, euphols, and triterpenes, some molecules with low molecular weight
or short alkyl chains were also extracted from E. kansui; these include
derivatives of sterols and phenols, and vegetable acids (10). The
low-molecular-weight molecules show an even higher cytotoxic activity and
exhibited better antiproliferative and antitumor properties, compared with
those of high-molecular-weight polymers (11, 12). However, the inhibitory
activities of methyl esters with low molecular weight have not been
characterized, and the underlying mechanisms of antitumor activity and
apoptosis induced by E. kansui have remained largely unknown.
Apoptosis is a fundamental cellular activity to maintain the physiological balance of the organism and plays a necessary role as a protective mechanism against carcinogenesis by eliminating damaged cells or cells that proliferate excessively (13). Knowing the background level of apoptotic cells throughout the drug discovery and screening process becomes increasingly important, as recent studies have found that natural and synthetic compounds affect apoptosis. Chemoprevention and chemotherapy, including the use of natural products, synthetic compounds, or dietary substances, are promising ways to stop or reverse the process of carcinogenesis. In this study, the inhibitory activity of the methyl esters and derivatives isolated from E. kansui and their effect on apoptosis and cell cycle distribution in the human gastric cancer cell line (SGC-7901) were evaluated by using trypan-blue, MTT (3-(4,5-dimethyl thiazol-2yl)- 2,5- diphenyltetrazolium bromide), and FCM (flow cytometry) assays. 5-fluorouracile (5-FU), the most widely used chemotherapeutic drug in clinical practice, was used for a positive control.
MATERIALS AND METHODS
Plant Materials
The plant Euphorbia kansui was collected in Tianshui,
China, by the Gansu Provincial Medical Company in October 2003 and the voucher
specimen was retained in our laboratory for future reference. To increase the
overall extract yield, the air-dried roots of E. kansui (1 kg) were pulverized in a grinder and
dissolved in acetone for 20 min at 60 oC. After filtration, the
solid residue was soaked in 2% tartaric acid and incubated at room temperature for
30 min. The resulting fluid was extracted with ethanol-chloroform (1:1).
The extract was then mixed with 2% Na2CO3 and 100%
ethanol, concentrated under reduced pressure, and lyophilized to yield 127.3 g
light yellow extract. The extract was purified by HPLC method and the chemical constituents
were identified by gas chromatography-mass spectrometry (GC-MS).
Cell Cultures
The human gastric SGC-7901 cell line was
obtained from the Shanghai Institute of Cell Biology, the Chinese Academy of
Sciences, which provides SGC-7901 for research for a long time. Cells were routinely
maintained at 37 oC in 5% CO2 as subconfluent monolayers
in 20 ml culture flasks in the laboratory of Lanzhou Army Hospital in China. Prior
to treatment, the cells were cultured in RPMI 1640 medium supplemented with 10%
fetal bovine serum (FBS), 2% penicillin-streptomycin, and 1% L-glutamine overnight.
The experimental procedure was approved by the Chinese Academy of Medical
Sciences, China.
Biological Assays
Live-cell cultures are dynamic, and the
proportion of viable, dead, and apoptotic cells continuously fluctuates as a
culture grows. Several assays were employed to evaluate cell health at various
times during screening, including trypan-blue, MTT, and FCM. The solvent control contained dimethyl
sulfoxide (DMSO) at a concentration less than 0.003%. 5-FU and PBS (phosphate
buffered saline) were used as positive and negative controls, respectively.
1) Trypan-blue assay
To screen the proportion of apoptotic cells
during the time course of screening, cells at a concentration of 1.0*105 cells/ml
were plated into 5 disposable 96-well culture plates, containing 180µl of
growth medium per well. After 24 h incubation at 37 oC in an
incubator supplemented with 5% CO2, 20 µl of PBS, 5-FU (10µg/ml), or
the extract of E. kansui at doses of 0.1, 1.0, and 10.0µg/ml were
applied to 16 wells of each plate,
respectively.
Plates were
incubated at 37 oC in an incubator supplemented with 5% CO2, and
were analyzed at 6, 12, 18, 24, and 48 h. For each well, after removal of the
supernatant, 20 µl of 0.01% trypsin, 160 µl RPMI 1640 medium, and 20µl of 0.4%
trypan-blue were then added. Cytotoxicity (the cellular growth inhibitory rate)
was determined from the number of viable cells (no color) in treated samples as
a percentage of the PBS control.
2) MTT colorimetric
assay
The MTT assay is
commonly applied as a preliminary screen to quantify cell proliferation,
viability, cytoxicity, and sensitivity (14, 15). The SGC-7901 cell line at a
concentration of 2.5*105 cells/ml was plated in a 96-well disposable
plate containing 180µl of growth medium (RPMI 1640) per well. After incubation
for 24 h at 37 oC in an incubator supplemented with 5% CO2,
20 µl of PBS, 5-FU (10 µg/ml), or the extract of E. kansui at doses of
0.1, 1.0, and 10.0 µg/ml were applied to 16 wells, respectively. The plate was
incubated for 48 h at 37 oC in an incubator supplemented with 5% CO2.
After the addition of 10 µl of MTT suspended in PBS (5 mg/ml) to each well, the
plate was incubated for further 4 h. Then the supernatant was discarded, 100 µl
solvent control (DMSO) was applied to each well, and the plate was shaken for
10 min. The optical density was read at 494 nm (OD494) in an
enzyme-linked immunodetector (MULTISKAN MK3, Shanghai, China), and the
inhibitory rate (IR) was calculated by the following formula:
Inhibitory
rate (IR) = (1 - average OD494 of treated group/average OD494 of
the PBS control) * 100%.
3) FCM assay
To quantify apoptosis by flow cytometry, cell
lines in this study were treated with propidium iodide and RNAase for DNA
fragmentation analysis. Cells at a concentration of 2.0*106 cells/ml
were inoculated into five 20ml-culture-flasks containing 4 ml of RPMI 1640 medium
supplemented with 10% FBS, 2% penicillin-streptomycin, and 1% L-glutamine. After
incubation for 24 h at 37 oC in a humidified incubator containing 5%
CO2, the supernatant was discarded, and 500 µl of 5-FU (10µg/ml),
PBS, or the extracts of E. kansui (0.1, 1.0, and 10.0µg/ml) were added
to the culture flasks, respectively. After 48 h incubation, the supernatant was
removed, and 2 ml of 0.01% trypsin were added to separate monolayer cells. After
treatment, cells were collected by centrifugation at 1,000 rpm for 5 min, fixed
with 75% cold ethanol, and incubated for 24 h at 0-4 oC. After
removal of ethanol, cells were resuspended in PBS containing 50 mg/ mL RNase A and 10 mg/mL propidium iodide, followed by incubation at
37 oC for 1 h. Apoptosis was analyzed by flow cytometry (FCM) (Coulter EPICS XL, US).
Statistical Analysis
In the statistical analysis, differences between the treated and the PBS groups were compared using Student's t-tests, with differences at the p<0.05 level considered statistically significant.
RESULTS
Six methyl esters or derivatives were isolated
from the extract of E. kansui, and their chemical structures and
compositions were identified by a gas chromatography-mass spectrometry (Figure
1). The Methyl ester derivatives
include (A) 11,13-eicosadienoic acid methyl ester (23.5%); (B) 12-octadecenoic
acid methyl ester (21.6%); (C) (Z, Z)-methyl ester-9,12-Octadecadienoic acid (17.5%);
(D) 10-methyl-heptadecanoic acid methyl ester (13.1%); (E) hexadecanoic acid methyl
ester (11.5%); and (F) methyl ester –5-oxo-DL-proline (11.4%). Subjecting the
SGC-7901 cells to the extract of E. kansui confirmed that these methyl
ester derivatives could initiate growth inhibition of the SGC-7901 cells and
induce apoptosis in a dose- and time-dependent manner. Their inhibitory effects
are comparable to those of the common antitumor agent 5-FU.
The extract of E.
kansui stimulated cellular proliferation and decreased cellular viability
as determined by the MTT assay; this effect was dose-dependent. After 48 h
incubation with the E. kansui extract at doses of 0.1, 1.0, and
10.0µg/ml, the inhibitory rates (IR) for tumor cells were increased by 36.3%, 70.7%, and 85.9%,
respectively (Figure 2). The 50% inhibition (IC50) was 0.265µg/ml.
Viability assays measure the percentage of a cell suspension that is viable. When the cell line was subjected to the trypan-blue assay, the extract treatment resulted in a significant reduction in the average number of tumor cells at 48 hours. The cellular growth inhibitory rates were increased by 28.5%, 62.2%, and 73.5%, respectively (Figure 3), in a time-dependent manner. The IC50 was 0.928µg/ml.
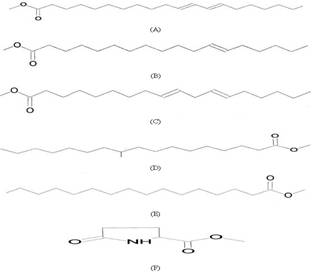
Figure
1: Chemical structures of the methyl esters and
derivatives isolated from Euphorbia kansui. (A) 11, 13-eicosadienoic
acid methyl ester. (B) 12-octadecenoic acid methyl ester. (C) (Z,
Z)-methyl ester-9, 12-Octadecadienoic acid. (D) 10-methyl-heptadecanoic
acid methyl ester. (E) hexadecanoic acid methyl ester. (F) methyl
ester –5-oxo-DL-proline.
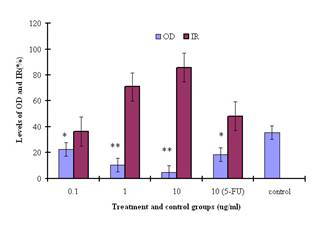
Figure
2: Inhibition of SGC-7901 cells by the methyl esters and
derivatives at concentrations of 0.1, 1.0, and 10.0µg/ml, assessed by the MTT
assay and expressed as a percentage of the PBS control values. * and ** indicate
statistically different from the control at P<0.05 and P<0.001 level,
respectively.
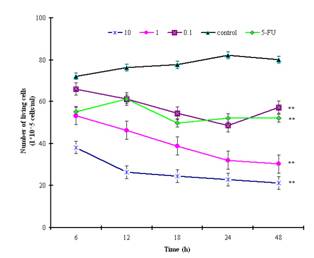
Figure
3: Proliferative inhibition of SGC-7901cells by the
methyl esters and derivatives assessed by the trypan-blue assay and expressed
as a percentage of the PBS control values. Cells that proliferated in 96-well
plates were incubated with different concentrations (0.1, 1.0, and 10.0µg/ml)
of the extract for various time intervals. ** indicates statistically
significant (P<0.001) differences from control.
Apoptosis is marked by a series of characteristics, such as loss of cell volume, clumping of chromatin and nuclear fragmentation into apoptotic bodies. Measurement of DNA content makes it possible to identify apoptotic cells and to recognize the cell cycle phase specificity of the apoptotic process (16). The effect of methyl esters and derivatives on the cell cycle progression of the SGC-7901 cells was confirmed by flow cytometry. As shown in Figure 4, compared with the PBS control, the methyl ester derivatives effectively inhibited the proliferation of SGC-7901 cells by interfering with the progression of cells through the G1 phase of the cell cycle. A sub-peak of apoptotic cells with high DNA content values was observed before the G1 period, and the cellular apoptosis rates were enhanced markedly by 23.3%, 28.8%, and 38.2%, when the cells were treated at concentrations of 0.1, 1.0, and 10.0µg/ml, respectively
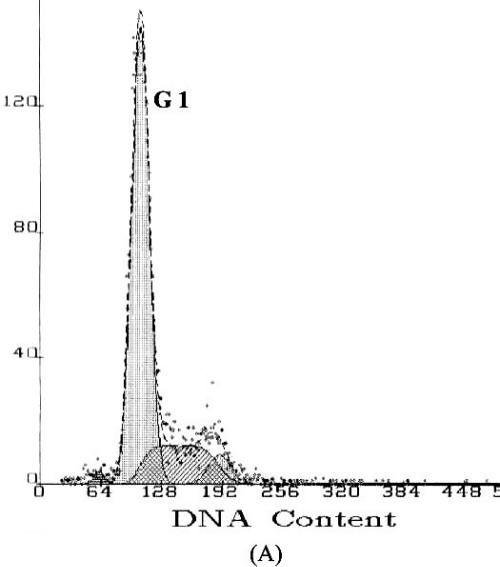
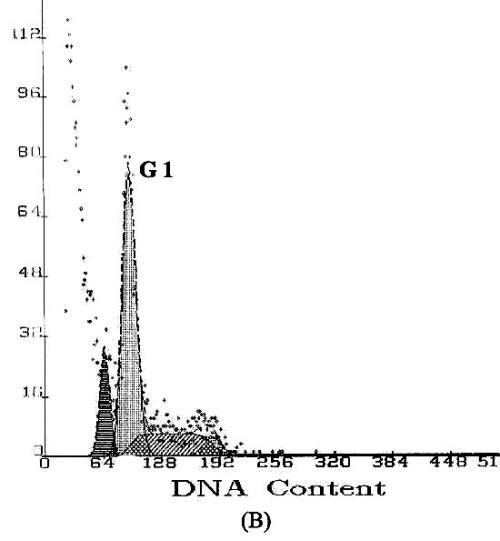
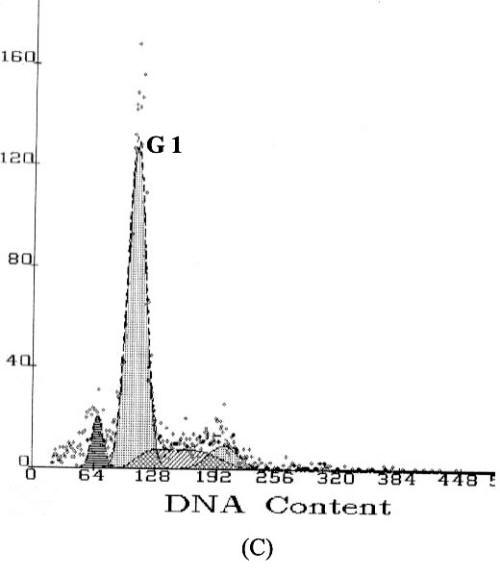
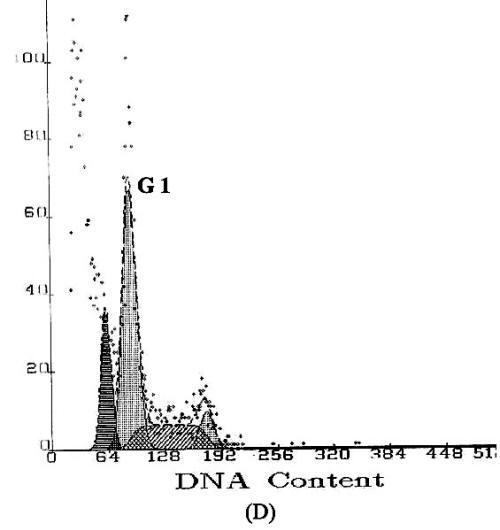
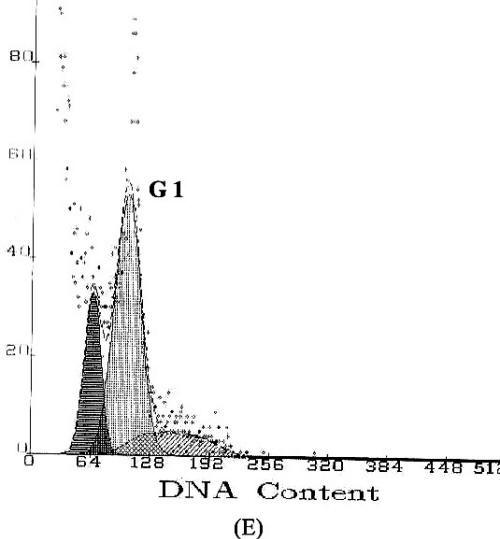
Figure
4: DNA content frequency histograms of SGC-7901cells
with treatment of the methyl esters and derivatives. The Y-axis is the number
of cells. Cells were fixed with ethanol and stained with propidium iodide, and
then cell cycle distribution was analyzed by flow-cytometry. (A) Cell
cycle following treatment with PBS. (B) Cell cycle following treatment
with 5-FU. (C–E) Cell cycles following treatment with the E.
kansui at concentrations of 0.1, 1.0, and 10.0µg/ml, respectively.
DISCUSSION
A large number of plant extracts of various
species of the genus Euphorbia (Euphorbiaceae) are used for the
treatment of various diseases in traditional Chinese medicine (6, 8, 14-17).
Previous studies have reported that the extract of E. kansui has an
antitumor effect on neoplastic cells, as well as inhibiting proliferation and
stimulating differentiation in multiple cancer cell lines, such as non-small
cell lung cancer, colon cancer, melanoma, and renal cancer (2, 3, 9, 15, 18).
Our study showed that methyl ester derivatives isolated from E. kansui,
including several common aliphatics such as hexadecane, heptadecane,
octadecane, and eicosane, possess antitumor activity and exhibit potent cytotoxicity
in the human cancer screening program. When the methyl ester compound was
applied to human gastric cancer cells, the inhibitory and apoptosis rates were
significantly increased (Figures 2 and 3).
Methyl esters and
derivatives isolated in this study are low-molecular weight polymers and are
expected to confer a relatively high hydrophilicity to molecules, one factor that
might be responsible for the enhancement of cytotoxicity (12). Our results
clearly demonstrate a cause-effect relationship between vegetable acids and
cytotoxic effect on tumor cells. When the cell
line was subjected to the trypan-blue assay, the methyl ester treatment
resulted in a significant reduction in the average number of tumor cells. The cellular
growth inhibitory rates were increased by 28.5-73.5% in a time-dependent manner
(Figure 3). At 48 h, the methyl ester treatment exhibited the greatest potency
against cells at a concentration of 10.0µg/ml and had a greater effect than
5-FU at the same dose. Similar antitumor-promoting and cytotoxic effects were
also observed with other methyl ester derivatives (15). More recent evidence
indicates that aromatic amino acid methyl esters resulted in high cytotoxic
effects against MCF-7 cells (19); betulinic acid possesses a broad spectrum of
activity against cancer cell types, including anti-HIV activity, cytotoxicity,
and antitumor properties (20, 21).
The typan-blue
method is a technique using dye exclusion, whereby cells with an intact
membrane are able to exclude the dye while cells without an intact membrane
take up the coloring agent. Because all of the compounds extracted are highly
lipid soluble, it may be possible that these compounds exert their effects via
interaction with the membrane that change membrane fluidity and
indirectly alter the Na+ current, resulting in disrupted membrane
integrity. For instance, octadecenoic acid, which has been found to be a
mechanism-based inhibitor of lipoxygenase, has been reported to be toxic in
humans by blocking both the Na+ current and the transient outward K+
current (22).
Metabolic and ionic
changes in cell culture are often associated with tumor cell proliferation,
malignant characteristics, and loss of apoptosis. It has been reported that the
treatment of euphane and tirucallane triterpenes resulted in an inhibition of
proliferation and an induction of apoptosis, which are regarded as the
preferred ways to manage cancer (9, 23). Further support for the observation of
cytotoxicity of E. kansui to tumor cells came from the present
experiments with the MTT assay. Our findings indicated that the decreased tumor
cell growth was elicited by methyl esters. At hour 48, the inhibitory rate of
tumor cell proliferation was increased by 36.3-85.9% in the cells treated with
methyl ester extract. The cellular apoptosis rates determined by the
flow cytometry assay were also enhanced significantly, by 23.3-38.2%. The
maximal effect on proliferation inhibition was observed at a concentration of 10.0µg/ml,
which is much better than that in antitumor activity of 5-FU.
Cancer is frequently
considered a disease of the cell cycle. The apoptosis of tumor cells induced by
methyl ester compounds can also been explained in terms of DNA degradation.
Studies have verified the antiproliferative specificity of the methyl ester
derivatives on proliferation of some tumor cell lines (11, 12, 24-27) and
induction of apoptosis (28). The high molecular weight polymers isolated from E.
kansui, including polycyclic- and ingenane-type diterpenes and euphane-type
triterpenes, have antiproliferative and antitumor properties and significant
cleavage arrest activity for cell division (9, 29). When tumor cells (SGC-7901)
were treated with the extract, methyl ester compounds may interact with the
cell membrane to alter permeability characteristics and then affect the entry
or exit of amino acids and nucleotides known to regulate cellular metabolism
(15), and thus result in cellular structural changes simultaneously with their
functional changes in both physiological and pathological conditions. This
effect implies that the methyl ester-induced disruption could functionally and
structurally damage cell membrane as well as other cellular structures and
ultimately cause cell death.
The formation of distinct DNA fragments is a biochemical hallmark of apoptosis, with internucleosomal DNA cleavage activity as a major characteristic (30). The normal metabolic cellular activities of the G1 period in cell division are in preparation for mitosis, including transcription, translation, and increase of cytoplasmic materials. The flow cytometry assay presented here suggests a possible association between methyl esters and cell cleavage arrest activity. As shown in Figure 4, the methyl ester extract apparently affected the proliferation of SGC-7901 cells by interfering with the progression of the SGC-7901 cells through the G1 phase of the cell cycle. When tumor cells are treated with methyl ester compounds, apoptotic cells with high DNA content in the treated groups apparently accumulate during the G1 period, in comparison with the PBS control (Figures 4C to E). As a result, the synthesis of proteins involved in transcriptional regulation and cell cycle control and the completion of the S and M phases are delayed, giving rise to a plethora of cellular effects, not least of which is potential activation of pathways leading to cell cycle arrest and apoptosis. The DNA fragmentation and DNA degradation in G1 phase were also observed when the human leukemic HL-60 cell line was treated with Genistein (16). It is thus assumed that the antiproliferative properties demonstrated by the methyl ester extract are attributed to their ability to penetrate the nucleus and interact with nuclear targets, leading to DNA degradation, unscheduled proliferation, and possibly latent DNA replication in dividing cell.
CONCLUSION
Our study has clearly demonstrated that methyl esters and derivatives may be promising chemopreventive agents for treating various forms of cancer by causing apoptosis and proliferation inhibition. As this species has not been previously screened against the cell line deployed in the present study, the present data are novel. However, the mechanism by which methyl ester analogues induce apoptosis in the cancer cell lines is as yet not completely understood, and it is still unclear how diverse cellular processes are coordinately deregulated in human cancer. An integration of phytomedical and biochemical studies will undoubtedly help extend this knowledge to therapeutic approaches.
ACKNOWLEDGEMENTS
We would like to thank Laurie Wilkins (Florida
Museum of Natural History, USA), whose comments and corrections of manuscript
significantly improved the content of this paper. Support for this research was
provided by a grant from the Gansu Institute of Political Science and
REFERENCES
[1] Daisuke U, Yoshimasa H. The structure of
kansuinine A, a new milt-oxygenated diterpene from Euphorbai kansui.
Tetrahedron Lett, , 21:1697-1700, 1975.
[2] Wu TS, Lin YM, Haruna M, Pan DJ, Shingu
T. Antitumor agents, 119. Kansuiphorins
A and B, two novel antileukemic diterpene esters from Euphorbia kansui.
J Nat Prod, 54:823-829, 1991.
[3] Yu FR, Hou XL, Chen J. Study of inhibition and antioxidation of Mao
Eryan extract on L615 leukemia cell. J Lanzhou Univ, 39:453-460,
2003.
[4] Wu FY, Haruna M, Lu XS. An experimental study on bacteria and
endotoxin translocation and kansui treatment in early acute hemorrhagic
necrotic pancreatitis. J China Modern Med, 6(5):7-9, 1996.
[5] Yasukawa K, Akihisa T, Yoshida ZY. Inhibitory effect of euphol, a triterpene
alcohol from the roots of Euphorbia kansui, on tumor promotion by
12-o-tetradecanoylphorbol- 13-acetate in two-stage carcinogenesis in mouse
skin. J Pharm Pharmacol, 54(1):119-124, 2002.
[6] Toth-Soma LT, Gulyas S, Szegletes Z. Functional connection between intracellular
and extracellular secretion in species of Euphorbia genus. Acta Viol
Hung, 44(4):433-443, 1993.
[7] Lin JH, Ku YR, Lin YZ, Teng SF, Wen KC,
Liao CH. Preparative isolation and gas
chromatography-mass spectrometry analysis of trterpenoids in kansui
radix. J Food Drug Anal, 8(4):278-282, 2000.
[8] Hohmann J, Molnar J, Redei D, Evanics F,
Forgo P, Kalman A, et al. Discovery and
biological evaluation of a new family of potent modulators of multidrug
resistance: reversal of multidrug resistance of mouse lymphoma cells by new
natural jatrophane diterpenoids isolated from Euphorbia species. J Med
Chem, 45(12):2425-2431, 2002.
[9] Wang LY, Wang LN, Yao XS, Miyata S,
Kitanaka S. Euphane and tirucallane
triterpenes from the roots of Euphorbia kansui and their in vitro
effects on the cell division of Xenopus. J Nat Prod, 66:630-633, 2003.
[10] Ding Y, Jia Z. Two phenolic derivatives from Euphorbia
kansui. Phytochemistry, 31:1435-1436, 1992,
[11] Bittoun P, Avramoglou T, Vassy J, Crepin
M, Chaubet F, Fermandjian S.
Low-molecular-weight dextran derivatives (f-CMDB) enter the nucleus and
are better cell-growth inhibitors compared with parent CMDB polymers. Carbohydr
Res, 322(3-4):247-255, 1999.
[12] Jin G, You Y, Ahn B. Esters of
2-(1-hydroxyalkyl)-1,4-dihydroxy-9,10-anthraquinones with melphalan as
multifunctional anticancer agents. Bioorg Med Chem Lett, 11(11):1473-1476,
2001.
[13] Hengartner MO. The biochemistry of apoptosis. Nature,
407:770-776, 2000.
[14] Betancur-Galvis LA, Morales FE, Forero
JE, Roldan J. Cytotoxic and antivriral
activities of Colombian medicinal plant extracts of the Euphorbia genus.
Mem Inst Oswqldo Cruz, 97(4):541-546, 2002.
[15] Whelan LC, Ryan MF. Ethanolic extracts of Euphorbia and
other ethnobotanical species as inhibitors of human tumor cell growth.
Phytomedicine, 10:53-58, 2003.
[16] Darzynkiewicz S, Bruno S, Del Bino G,
Gorczyca W, Hotz MA, Lassota P, et al.
Features of apoptotic cells measured by flow cytometry. Cytometry,
12:795-808, 1992.
[17] Madureira
AM, Ascenso JR, Valdeira L, Duarte A, Frade JP, Freitas G, et al. Evaluation
of the antiviral and antinicrobial activities of triterpenes isolated from Euphorbia
segetalis. Nat Prod Res, 17(5):375-380, 2003.
[18] Zheng WF, Chen CF, Zhu AH, Li MQ. Screening for antiviral fractions from
ethanol extract of Euphorbia kansui. J Chin Tradit Pat Med, 24(5):362-365,
2002.
[19] Iyer VV, Griesgraber GW, Radmer MR,
McIntee EJ, Wagner CR. Synthesis, in
vitro anti-breast cancer activity, and intracellular decomposition of amino
acid methyl ester and alkyl amide phosphoramidate monoesters of
3'-azido-3'-deoxythymidine (AZT). J Med Chem, 43(11):2266-2274, 2000.
[20] Urban M, Sarek J, Klinot J, Korinkova G,
Hajduch M. Synthesis of A-seco
derivatives of betulinic acid with cytotoxic activity. J Nat Prod,
67(7):1100-1105, 2004.
[21] Mukherjee R, Jaggi M, Siddiqui MJ,
Srivastava SK, Rajendran P, Vardhan A, et al.
Synthesis and cytotoxic activity of 3-O-acyl/3-hydrazine
/2-bromo/20,29-dibromo betulinic acid derivatives. Bioorg Med Chem Lett, 14(15):4087-4091, 2004.
[22] Harrell MD,
Stimers JR. Differential effects of linoleic acid metabolites on
cardiac sodium current. J Pharmacol Exp Ther, 303 (1):347-355, 2002.
[23] Matsumoto T, Cyong JC, Yamada H. Stimulatory effects of ingenols from Euphorbia
kansui on the expression of macrophage Fc receptor. Planta Med, 58(3):255-258,
1992.
[24] Rossi T, Castelli M, Zandomeneghi G,
Ruberto A, Benassi L, Magnoni C, et al.
Selectivity of action of glycyrrhizin derivatives on the growth of MCF-7
and HEP-2 cells. Anticancer Res, 23(5A):3813-3818, 2003.
[25] Banno N,
Akihisa T, Tokuda H, Yasukawa K, Higashihara H, Ukiya M, et al. Triterpene
acids from the leaves of Perilla frutescens and their antiinflammatory
and antitumor-promoting effects. Biosci Biotechnol Biochem, 68(1):85-90, 2004.
[26] Serrano A,
Palacios C, Roy G, Cespon C, Villar ML, Nocito M, et al. Derivatives
of gallic acid induce apoptosis in tumoral cell lines and inhibit lymphocyte
proliferation. Arch Biochem Biophys, 350:49-54, 1998.
[27] del Sol
JM, Garzon SP, Rodriguez AD. Plakortides M and N, bioactive polyketide
endoperoxides from the Caribbean marine sponge Plakortis halichondrioides.
J Nat Prod, 66(5):655-661, 2003.
[28] Kim KK, Kawano Y, Yamazaki Y. A novel porphyrin photosensitizer from bamboo
leaves that induces apoptosis in cancer cell lines. Anticancer Res,
23(3B):2355-2361, 2003.
[29] Uemura D, Hirata Y. New diterpene, 13-oxyingenol, derivative isolated
from Euphorbia kansui Liou. Tetrahedron Lett, 29:2529-2532, 1974.
[30] Bortner CD, Oldenburg NBE, Cidlowsk JA. The role of DNA fragmentation in apoptosis. Trends in Cell Biol, 5: 21-26, 1995.
Published by the Canadian Society for Pharmaceutical Sciences.
Copyright © 1998 by the Canadian Society for Pharmaceutical Sciences.