J Pharm Pharmaceut Sci (www.cspscanada.org) 8(3):602-625, 2005
Drug Disease Interactions: Role of inflammatory mediators in disease and variability in drug response
Kenneth M. Kulmatycki1, Fakhreddin Jamali2
1Drug Metabolism and Pharmacokinetics,
Schering-Plough Research Institute,
2Faculty of Pharmacy and Pharmaceutical Sciences,
Received October 11, 2005; Revised December 13, 2005; Accepted December 14, 2005; Published December 16, 2005
ABSTRACT Expression of both pro- and anti-inflammatory mediators are influenced by various factors such as rheumatic diseases, myocardial infarction, angina, aging, obesity and pharmacotherapy. This has therapeutic consequences. Clearance of highly bound and efficiently metabolized drugs may be reduced in the presence of inflammation amounting to increased circulating drug concentration. In the meantime, various cardiovascular receptors are down-regulated in the presence of pro-inflammatory mediators. Consequently, conditions such as rheumatoid arthritis, aging and obesity results in reduced response to drugs such as verapamil despite increased drug concentration. The inflammatory response is a complex cascade of non-specific events resulting in excessive generation of inflammatory mediators such as cytokines, C-reactive protein and nitric oxide by cells of the innate (macrophages, monocytes, neutrophils) and adaptive (T-lymphocytes) arms of the immune system. T-lymphocytes secrete various pro- and anti-inflammatory cytokines during an inflammatory event. In general, two distinct subpopulations of these T-helper cells exist, anti-inflammatory Th2 and pro-inflammatory Th1. As a common rule, Th1 cytokines suppress Th2 and vice-versa. Hence, a balance of these activities is desired. Drugs such as antirheumatoid agents, angiotensin II blockers and hydroxymethyl-glutaryl-CoA reductase inhibitor (statin) may help to restore the Th1/Th2 balance. In general, at least for some conditions, the challenge of therapeutic drug monitoring will be more useful if expression of inflammatory mediators is also taken into account. In addition, some of the intersubject variation in pharmacotherapy and clinical trails may be attributed to variations in the inflammatory mediator’s concentration. A detail list of conditions and drugs that influence expression of the inflammatory mediators are provided and potential therapeutic consequences are discussed.
The inflammatory response is a complex cascade of non-specific events stimulated by infection or tissue damage (1). This usually is a well-controlled process. However, excessive generation of inflammatory mediators such as cytokines, C-reactive protein (CRP) and nitric oxide (NO) by cells of the innate (macrophages, monocytes, neutrophils) and adaptive (T-lymphocytes) arms of the immune system can result in disease (1, 2). T-lymphocytes (cells) secrete various pro- and anti-inflammatory cytokines during an inflammatory event resulting in outcomes ranging from elimination of pathogens to allograft rejection (3-5). Involvement of T-cells during inflammation is dependent on the population of activated cells. Briefly, CD4+ T cells conduct adaptive immune responses in reaction to foreign antigens. In general, two distinct subpopulations of CD4+ T helper cells exist due to unique cytokine arrays produced. These are the T-helper 1 phenotype (Th1) and the T-helper 2 phenotype (Th2). Th1 cells predominantly produce IL-2, IFN-g and TNF-a. These cytokines induce cellular immune responses and activate macrophages. The Th2 phenotype mainly produces IL-4, IL-5, IL-10 and IL-13 that are important in aiding B cell activation and antibody production. As a common rule, Th1 cytokines suppress Th2 and vice-versa. Thus, once a particular T helper cell immune response is established (Th1 or Th2), the polarized sub-type tends to persist through positive feedback mechanisms (6-11). Expression of specific cytokines by T-helper cells results in Th1 and Th2 cell dominant inflammatory disorders (12, 13).
Cytokines are
a diverse group of soluble messenger proteins involved in the activation,
growth, control and repair of cells and regulation of immune events (2, 9). Cytokines
may act within the same cell (autocrine), nearby (paracrine) or at distant
sites (endocrine) (1, 2). Strict regulation, transient production, and binding
to cell surface receptors normally occurs with redundancy and pleiotropy observed
(14, 15). The pleitropic nature results from many cells having receptors for
the same cytokine and the overlapping function of cytokines creates redundancy
leading to formation of networks with cascade responses. Sequential expression due
to cytokine cascades occurs in many conditions, for example, in rheumatoid arthritis,
IL-1, -6, -8 and granulocyte macrophage-colony stimulating factor are expressed
downstream of TNF-a (16,17). Other conditions in which cytokine cascades are reported are congestive
heart failure, multiple myeloma and sepsis (18-20). Cytokines e.g., IL-1, -2,
-6, -12, -18, TNF-a, -b (lymphotoxin),
interferon (IFN)-a, -g, and various chemokines
such as IL-8, regulation-upon-activation normal T expressed and secreted
(RANTES), macrophage inflammatory protein (MIP)-1a, MIP-1b are involved in progression of inflammatory events (21,22).
Anti-inflammatory cytokines e.g., IL-4, -5, -10, -13 generally counteract the
cellular activation and production of proinflammatory cytokines and involved in
immunity and allergic reactions (2, 8). Chemokines are low-molecular weight
cytokines that can mediate migration of leukocytes during inflammation for
example CXCR1, CXCR2, CCR2 and CCR3 regulate leukocyte trafficking to tissue
sites of inflammation (23, 24).
Inflammation can be acute and limiting or chronic. The latter may be due to a persistent antigen resulting in conditions such as rheumatoid arthritis. Acute inflammation may lead to a systemic reaction known as the acute phase response in which stimulated macrophages secrete TNF-a, IL-1 and IL-6 which act on the hypothalamus to produce fever and on the liver to induce production of acute phase proteins (e.g., a1-acid glycoproteins and CRP) by hepatocytes (25). In addition, TNF-a acts on vascular endothelial cells and macrophages to increase secretion of colony stimulating factors (e.g., macrophage-colony stimulating factor) that stimulate hematopoiesis resulting in increased white blood cells to fight infection and immune cells such as activated macrophages to secrete hydrolytic enzymes and reactive nitrogen products (26). Nitric oxide (NO) is a soluble gas that participates in normal physiological processes such as vasodilation and neurotransmission; however, overexpression may result in disease as observed in asthma, cardiovascular disorders and organ transplant rejection (27-29). NO is generated from L-arginine which is catalyzed by a group of enzymes called nitric oxide synthases (NOS): constitutive forms [endothelial NOS (eNOS), neuronal NOS (nNOS)] and inducible NOS (iNOS)] (30). Toxicity emerges when excessive concentration of NO is expressed. Alterations in constitutive forms (e.g., eNOS) may also cause disease (31). Unlike constitutive forms, iNOS activity is induced by proinflammatory cytokines (e.g., IFN-g, IL-1b and TNF-a) and participates in events that lead to inflammatory disorders (18, 26, 32). Research in the area of inflammatory mediator overexpression (e.g., cytokines, NO) has been evolved from discovering and cataloging to determining roles in disease (33).
We have highlighted the therapeutic significance of altered inflammatory mediators in a review article published in 2001 (12). Since then, however, the interest on the topic has greatly increased as reflected in the body of the published reports. Hence, the intention of this review article is to update the readership with the most recent advances in the field.
Acquired
immunodeficiency disorder (AIDS), asthma, atherosclerosis, cancer, congestive
heart failure (CHF), diabetes, rheumatoid arthritis and depression are examples
of disorders in which disease pathogenesis has been linked to increased
expression of proinflammatory mediators (17,21,34-44). In fact, inflammatory
diseases are a major cause of mortality, in the year 2000 diseases of the
heart, malignant neoplasms and diabetes accounted for over one-half of all
deaths reported to the Centers for Disease Control and Prevention (45).
Overexpression of proinflammatory
cytokines has also been associated with allograft rejection (46). For example, IFN-g, sIL-2 receptor, IL-4 and IL-10 serum concentrations were
compared in 105 infants and children after liver transplantation with and
without acute graft rejection episodes. The incidence of acute rejection by age
groups was 0-12 months (26.8%), 1 to 3 years (40%) and children greater than 3
years old (71.8%). There was significantly lower incidence of acute rejection episodes
in infants up to 12 months of age compared to those greater than 1 year old.
Analysis of serum Th1 and cytokine expression up to 24 months from children with
and without rejection episodes is shown in
Figure 1. Between the two groups,
increased expression of IFN-g and sIL-2 receptor was associated with acute rejection episodes. Except
four weeks post-transplantation, cytokine patterns did not differ significantly
from preoperative values in both groups. Compared to healthy controls, patients
with and without acute rejection 12 months post-transplantation showed no
differences regarding the absolute number of T and B cells, T helper cells,
cytotoxic T cells and activated (HLA-DR+) T cells, thus, differences between
the two groups was not due to changes in populations of B- and T-cells.
Increased Th2 expression i.e., anti-inflammatory cytokines IL-10 and IL-4 of
infancy was concluded to be an important factor in reducing acute rejection
episodes (46). Disorders that have been associated with proinflammatory
mediator overexpression are shown in
Table 1.
Disease due to overexpression of mediators
of inflammation may also result from cytokine treatment or concurrent disorder.
For example, patients afflicted with renal cell carcinoma, melanoma or
hepatitis C virus infection, and administered IL-2 or IFN-a2b have been reported to experience depressive
symptoms (185-187). In addition, rheumatoid arthritis is reported to be an
independent risk factor for occurrence of cardiovascular disease (188,189).
Cachexia, a wasting syndrome characterized by loss of fat tissue, skeletal
muscle, bone tissue and anorexia, is another example of a disorder that may
occur with other inflammatory diseases. This wasting syndrome is characterized
by proinflammatory mediator overexpression (e.g., TNF-a) and contributes to mortality (190,191). Increased
expression of proinflammatory mediators and wasting has been reported for
patients afflicted with CHF, cystic fibrosis, tuberculosis and cancer
(118,119,192-196). A prospective study that was conducted in which the
frequency and prognostic importance of cachexia in patients with CHF was
determined (120). Anker et al assessed 171 consecutive patients afflicted with
CHF and discovered that 28 of these patients were cachectic. Compared to
non-cachectic patients these individuals were slightly older, had reduced
exercise capacity and time. They also had lower sodium plasma concentrations.
Their left ventricular ejection fraction was, however, similar in both groups
of patients. An 18-month follow-up of these patients was conducted in which
all-cause mortality was the endpoint. The cachectic state was predictive of
mortality at 18 months independent of age, CHF classification, left ventricular
ejection fraction, peak oxygen consumption and sodium levels. Congestive heart
failure patients who had two risk factors such as reduced oxygen consumption
(less than 14 ml/min/kg) and cachexia had an extremely poor survival compared
to patients that did not have these two risk factors. Mortality in CHF patients
with cachexia was very high: 18% at 3 months, 29% at 6 months and 50% at 18
months compared to those without cachexia as shown in
Figure 2.
Similarly,
in cancer patients the altered balance between proinflammatory and anti-inflammatory
cytokines is considered a major determinant of progression to cancer cachexia
and reported to account for almost one-third of cancer deaths (190,191,196).
Normalizing inflammatory responses in cachectic patients may help to increase
the effectiveness of various treatments. For example, modifying proinflammatory
cytokine concentrations may improve the effectiveness of administered nutrients
in total parenteral nutrition, which may be of benefit to those receiving
parenteral nutrition afflicted with cachexia (197).
Altered
proinflammatory mediator expression occurs with aging. Increased concentrations
of IL-6 in older persons are linked to development of depression and
atherosclerosis. In fact, overexpression of IL-6 and CRP in elderly individuals
has been associated with disease, disability and mortality (92,93,198,199). To investigate whether TNF-a overexpression is associated with cognitive function,
atherosclerosis and general healthy status, serum concentrations of TNF-a, sTNFRII (free and bound), IL-6 and CRP have been
determined in 126 centenarians, 45 (81 year-old), 23 (55-65 year-old) and 38
(18-30 year-old) individuals (91). Concentrations of TNF-a and TFNRII were greatest in centenarians and greater
in 80 year-olds compared to younger groups. Concentrations of IL-6 were greater
in 55-65 year-old, 80 year-old and centenarians than younger people. High TNF-a concentrations were associated with moderate to
severe dementia independent of atherosclerosis and Alzheimer’s disease.
Increased concentrations of TNF-a were correlated with IL-6, sTNFRII and CRP in the
centenarians showing that increased concentrations of inflammatory mediators
observed in aged individual is associated with development of a persistent
low-grade inflammatory state.
Overexpression of proinflammatory mediators in disease is reported to be linked to treatment failure. For example, increasing levels of IL-1ra and IL-6 during the first 2 days of hospitalization in unstable angina patients have been shown to be associated with increased risk of in-hospital coronary events (200).




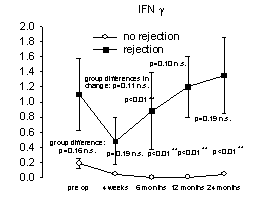
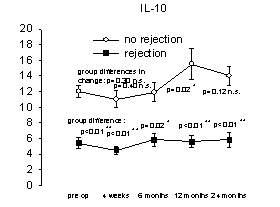
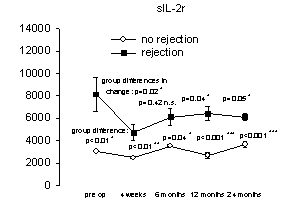
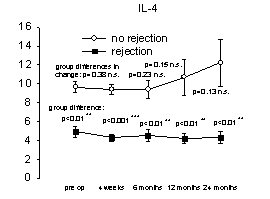
Figure 1. Relationship between
proinflammatory mediator overexpression and acute liver rejection episodes.
No differences were found within each group (no rejection versus rejection),
however, patients experiencing acute rejection episodes had increased
concentrations of proinflammatory cytokines/receptors (Th1) and decreased
concentrations of anti-inflammatory (Th2) cytokines [mean cytokine/receptor
concentration (pg/ml) versus pre- and post-operative transplantation,
(n=11/Group)]. From reference 46 with permission.
In further support, to determine whether the proinflammatory state independently determines outcome in patients with unstable angina, inflammatory markers were compared between 135 stabilized and 76 refractory patients. Standard medical therapy consisted of acetylsalicylic acid (ASA), nitroglycerine, heparin, β-adrenergic blockers and calcium channel blockers.
Refractory patients had higher serum concentrations of CRP, fibrinogen and erythrocyte sedimentation rate compared to stabilized patients, which was not affected by presence or absence of myocardial necrosis (measured by troponin-T), or interval between onset of angina and blood collection. In severe unstable angina, the proinflammatory state was determined to be the main independent determinant for short term therapy failure (201).
CRP overexpression has been associated with many cardiovascular disorders and suggested to be predictive of treatment failure (202-205). In fact, CRP and the high-sensitivity CRP (hs-CRP) assays have provided benefit in determining relationships between the mediator overexpression and disease, effectiveness of pharmacotherapy, and outcome (206-209). Immunmodulatory effects of CRP in inflammatory disorders reported to date are upregulation of adhesion molecules, monocyte recruitment, and complement activation, interaction with lipids and thrombosis, and inhibiting NO production (74,210).
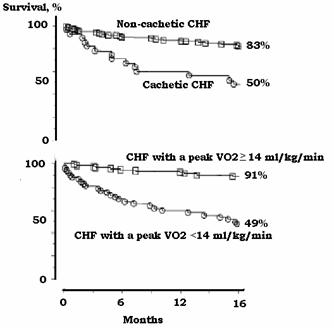
Figure 2.
Kaplan-Meier survival curves showing mortality of
cachetic patients with congestive heart failure (CHF). Mortality of cachetic
patients with CHF was similar to those that had low peak oxygen consumption (<14 ml/kg/min). Survival in ambulatory patients with
congestive heart failure (top frame) with (n=28) and without (n=143) cardiac
cachexia and (bottom frame) survival in these patients based on VO2
(oxygen consumption) with peak VO2<14 (n=53) and VO2>14 ml/kg/min (n=118). From reference
120 with permission.
Post-myocardial infarction patients with high CRP concentrations are more likely to have a history of unstable angina and symptom onset at lower levels of activity than those with lower concentrations emphasizing the role of CRP in disease progression (211,212). Pietila et al studied the relationship between serum CRP and mortality in patients who had experienced an acute myocardial infarction (213). CRP concentrations and creatine kinase and its MB isozyme were measured in 188 patients who had undergone an acute myocardial infarction. CRP concentrations were measured daily for 6 days after the infarction, serum creatine kinase and its MB isozyme were measured on admission and twice daily for 3 days. Mortality was determined at 3, 6, 12 and 24 months after the infarction and compared with CRP and creatine kinase values. Peak serum values of creatine kinase and its MB isozyme were similar in all patients and were not associated with mortality. On the other hand, increased concentrations of CRP in patients the first 2 days after an acute myocardial infarction were associated with increased risk of dying of cardiac failure or sudden death 6 months after the infarction as shown in Figure 3.
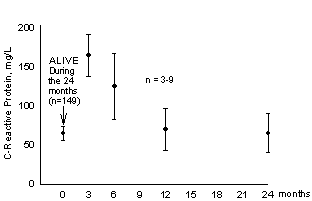
Figure 3.
Relationship between CRP serum concentrations measured
2-4 days after myocardial infarction and mortality. From
reference 213 with permission.
In addition, elevated CRP concentrations in patients at discharge are reported to be associated with recurrent instability in patients with unstable angina independent of coronary revascularization procedure (214). Increased CRP concentrations are also associated with cardiovascular risk factors in the elderly and predictive of overall and cardiovascular mortality (93,215-217). Similarly, measurements of TNF-a soluble receptors were associated with older age and increased levels of CRP that predicted treatment outcome in patients with Hodgkin’s disease and non-Hodgkin’s lymphoma (218). Increased inflammatory mediator expression and altered pharmacological response for various disorders are listed in Table 2.
Inflammatory conditions and inflammation-induced pathophysiological changes have been associated with increased plasma concentrations of some important drugs (249-251). Probable explanations for increased drug concentration are greater protein binding due to increases in a1-acid glycoproteins during the acute phase response and reduced metabolism due to NO and its breakdown products inactivating cytochrome P450 isozymes. (252-255). Contributions of increased protein binding and reduced metabolism depends on the role of metabolism and protein binding (251,256,257). Drugs that are highly protein bound and undergo extensive first pass metabolism are targets for inflammation-induced changes in pharmacokinetics. Substantial increases in plasma concentrations of propranolol, a highly protein bound and efficiently metabolized drug, are reported for patients afflicted with various inflammatory disorders (249).
Similarly, increased concentrations of verapamil enantiomers have been reported in patients with rheumatoid arthritis as shown in Figure 4 (230). This was accompanied by elevated IL-6 and serum nitrite (stable breakdown product of NO) concentrations compared to healthy volunteers. Verapamil similar to propranolol is highly protein bound and undergoes extensive first-pass metabolism thus elevated plasma concentrations were attributed to changes in both protein binding and hepatic metabolism. One would anticipate that higher concentration of verapamil in arthritic patients would result in increased activity. Quite the contrary, however, the observed elevated total and plasma unbound verapamil concentrations in arthritic patients were accompanied by a decreased dromotropic (L-type cardiac calcium cardiac channel) response demonstrated by a reduced prolongation of the cardiac PR interval as shown in Figure 4.
In support, data generated from elderly patients also demonstrate a reduced dromotropic activity of verapamil despite increased plasma concentrations (234). Furthermore, in elderly individuals β-blocker therapy does to appear to reduce risk of stroke or coronary events (236). In fact, b-blocker therapy is reported to be not efficacious as the first line therapy in hypertensive elderly patients (237). A reduced β-adrenergic responsiveness to propranolol in the presence of increased plasma concentrations using an animal model of inflammation has been observed (257). Plausible explanations for the reduced potency of verapamil in arthritic patients and propranolol in inflamed rat include increased protein binding of drug and/or altered function of L-type cardiac calcium channels and β-adrenergic receptors, respectively. Proinflammatory mediators have been shown in vitro to reduce activity of β-adrenergic, cardiac calcium and potassium channel receptor function due to down-regulation and/or inactivation of receptors and channels (258-263).
Reduced response to β-adrenergic antagonist has also been reported in the absence of pharmacokinetic alteration. Sotalol is a β-adrenergic as well as potassium channel blocker. It is negligibly protein bound, and undergoes little or no hepatic clearance. Inflammation, therefore, does not influence sotalol pharmacokinetics. Nevertheless, both β-adrenergic and cardiac potassium channel blocking activity of sotalol are reduced in the rat model of inflammation (264).
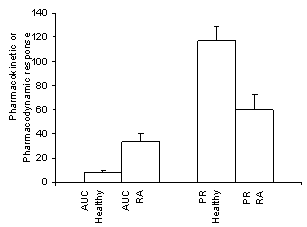
Figure 4.
Area under serum S-verapamil concentration-time
curves (AUC, mg/L-1 • min) and the area under percent PR
prolongation (from baseline, %Xh) following 80 mg of racemic verapamil to
healthy volunteers and rheumatoid arthritic patients (RA). Error bars represent
standard error of the mean (n=8/group). From reference 230
with permission.
The reduced response appears to be reversed with administration of anti-TNF-a monoclonal antibody, which is associated with reduced serum concentrations of TNF-a and nitrite. Similar observations have been made for atenolol (265) and propranolol (266). Interestingly, a recent report indicates that RA patients who are treated with anti-TNF-a antibodies demonstrate a lower incidence of first cardiovascular events (267). It should be noted that the use of anti-TNF therapy may also result in serious adverse effect. Although preclinical and preliminary clinical data suggested that they may favorably modify the course of disease, their use in New York Heart Association class III and IV heart failure and left ventricular ejection fraction may, indeed, adversely affected the clinical condition of patients with moderate-to-severe chronic heart failure particularly in high doses (268).
The reduced dromotropic activity of b-adrenergic, calcium channel and potassium channel antagonists appear to be associated with increased expression of proinflammatory mediators, and independent of pharmacokinetic alterations. The available evidence points to reduced binding of the drugs to the target proteins at the receptor level as shown in Figure 5 (269). The reduction in binding may be attributed to gene downregulation resulting in reduced mRNA synthesis or posttranslational alteration of receptor. Not all cardiovascular receptors are down-regulated by inflammation. At least for the angiotensin II type 1 receptors (AT1R) antagonist, valsartan, rheumatoid arthritis appears to have no down-regulating effect. Indeed, a trend toward an up-regulation is evident (270). This does not appear to be pharmacokinetic-dependent. A probable explanation for the trend towards increased potency may be upregulation of AT1Rs in inflammatory conditions due to the anti-inflammatory nature, and effect on the Th1 cells of this class of drugs (11,271-274). Indeed, angiotensin by itself has been identified as an endogenous compound with inflammatory properties (11). This may make AT1R antagonists as cardiovascular drugs of choice in the treatment of patients with inflammatory diseases.
Altered pharmacokinetics
and/or pharmacodynamics have been reported for many drugs including some used
to treat cardiovascular diseases, asthma, cancer, diabetes and depression. In
addition, conditions such as old age and obesity appear to be associated with
alteration of action and disposition of some drugs. All these are associated
with altered inflammatory mediators. Whether this association is causative or
incidental is, at most, controversial and remains to be unequivocally
understood. Nevertheless, altered concentration of inflammatory mediators appears
to influence pharmacokinetic-pharmacodynamic relationships. This may render the
conventional drug effect vs. concentration and/or dose-response relationships
more complicated as the mediators’ concentrations may have to be considered in
the equation. Hence, equations describing effect-concentration relationships
may have to be expanded from the present two dimensional to multidimensional
ones. For example, monitoring of inflammatory mediators in cancer patients has
been suggested to identify patients with very poor benefit-risk ratios for drug
treatment due to the association of inflammation with cancer, altered drug
metabolism, and reduced response to chemotherapy (275). Changes in drug
response are especially relevant to the elderly who have increased incidence of
concurrent conditions in conjunction with proinflammatory mediator
overexpression that occurs with aging. In fact, depressive symptoms in elderly
individuals are reported to constitute an independent risk factor for
development of coronary heart disease and mortality (276). Understanding the
influences of proinflammatory mediator overexpression on drug disposition and
activity, therefore, may help to explain variability in response to
pharmacotherapy.
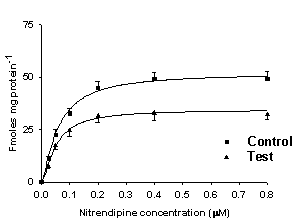
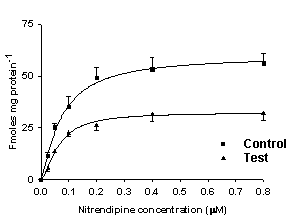
Figure 5.
Binding of 3H-nitrendipine
to cardiac cell membranes of rats with acute (left) and chronic (right) inflammation.
Binding (Bmax) was reduced in
rats with inflammation compared to controls (acute: control, 63.2±2.5; interferon
treated, 46.4±2.0; chronic:
control, 66.8±2.2, adjuvant
treated, 42.2±2.0 fmolmg-1
protein). In chronic inflammation increase in dissociation constant (KD) compared to normal rats was observed (normal, 0.09±0.01; treated, 0.14±0.02 µM). From Reference 269 with
permission.
Table 1: Proinflammatory
mediator overexpression and disease
|
Disorder |
Inflammatory Mediator Expression |
|
Acquired Immunodeficiency
Syndrome (AIDS) |
Increased secretion of TNF-a, IL-1 and IL-6 by macrophages and monocytes correlated
with viral load and polymorphisms in chemokine receptor and gene expression
is suggested to be associated with disease susceptibility and progression
(47, 48); increased capacity of dendritic cells exposed to HIV-1 to produce
TNF-a and IL-1b (47, 48); upregulation of CCR5 chemokine receptor
(21, 47-49); IL-10 overexpression contributes to B-cell hyperactivity and
risk of AIDS-lymphoma (50). |
|
Acute Infection |
Elevated myeloperoxidase and IL-6 in severe infections served as a
distinction between viral and bacterial causes (51). |
|
Atopic Diseases |
Promotion of eosinophilia and cytokines that regulate IgE in atopic
diseases including asthma, allergic rhinitis and atopic dermatitis (52,53);
allergic states and IL-4, IL-5, IL-10 and IL-13 were associated via Th2
responses (53,54); administration of IL-12, IFN-a/g are suggested to alleviate atopic disease (53,55); increased NO in
exhaled air reflected airway inflammation in asthma patients (56); increased
IL-5 concentrations with subsequent eosinophil activation was involved with
pathogenesis of asthma, IL-5-activated eosinophils downregulate IL-5 membrane
receptor and release soluble IL-5 receptor blunting subsequent IL-5-dependent
inflammatory events complicating treatment (57). |
|
Behçet’s Syndrome |
Active disease was associated with increased IL-6, -10, -17, -18 and
IFN-g compared to remission
(58). |
|
Cancer |
Increases IL-6 and IL-6sR are associated with progression and
metastasis of prostate cancer (59); elevated serum levels of IL-6 and sIL-6
receptor correlated with lower life expectancy in patients with multiple
myeloma (60); increased IL-17 concentrations promoted angiogenesis and tumor
growth (61). |
|
Cardiovascular Disorders |
|
|
Acute myocardial infarction |
Elevated plasma IL-6 levels after acute myocardial infarction (62);
CRP localizes in infarcted heart tissue (63); sequential appearance of IL-1b and IL-6 in plasma of patients that experience an
acute myocardial infarction (64); TNF-a overexpression post-myocardial infarction is not
confined to the infarct or peri-infact zone but is also present in the tissue
contralateral to the infarct (65); increased serum CRP concentrations
predicted MI in patients with peripheral vascular disease severe enough to
require revascularization (66); high CRP levels prior to thrombolysis was
associated with reperfusion failure with unfavorable short and long term
prognosis (67). |
|
Atherosclerosis |
CRP is a strong predictor for future coronary events in healthy
individuals (68); increased endothelium concentrations of IL-1 and TNF-inducible
adhesion molecules P-selectin, E-selectin, VCAM-1 and intracellular adhesion
molecule (ICAM)-1 in atherosclerotic tissue (69,70); high density
lipoproteins may protect against coronary artery disease by inhibition of
adhesion molecules (71); high density lipoproteins are suggest to inhibit
TNF-a and IL-1b from increasing expression of E-selectin, VCAM-1
and ICAM-1 (36,69); endothelial dysfunction is associated with altered NO
bioavailability due to either reduced formation or accelerated degradation
(72); CRP levels predicted future risk of coronary heart disease in healthy
middle-aged men (73);CRP suggested to have a fundamental role in
atherogenesis (74). |
|
Congestive Heart Failure |
Increased concentrations of TNF-a and IL-6 were associated with progression from
asymptomatic to symptomatic left ventricular dysfunction and excessive TNF-a levels associated with mortality (38,75,76); IL-6
is a strong predictor of disease progression (77); patients without cachexia
that experience acute decompensation have increased levels of TNF-a (78). |
|
Hypertension |
Increased IL-1ra concentrations in essential hypertensive patients
compared to normotensive individuals (79); hypertensive type II diabetic
patients prothrombic state suggested to result in higher incidence of
thrombotic events compared to non-diabetic hypertensive patients (80); hsCRP
independent risk factor for hypertension (81). |
|
Unstable angina |
Imbalance between TNF-a and IL-10 reported for patients with unstable
angina (82); increased concentrations of markers of inflammation in unstable
angina (83); increased concentrations of CRP, macrophage colony-stimulating
factor (MC-SF) and IL-6 was related to number of diseased vessels and reduced
after six weeks of aspirin treatment (84); preprocedural CRP predicted early
complications and restenosis after coronary angioplasty (85); higher levels
of IL-10 were associated with reduced risk of coronary events in patients
with unstable angina (86); increased IL-6 serum concentrations independent
marker of increased mortality in patients with unstable coronary artery
disease (87). |
|
Stroke |
Reduced IL-10 concentrations were associated with neurological
worsening in patients that experienced ischemic stroke (88). |
|
Elderly |
Age associated changes in T-cell chemokine expression is suggested to
contribute to poor clinical outcome of T-cell chemokine receptor-dependent
diseases in the elderly (89); elevated concentrations of IL-1, TNF-a, IL-6 and sTNFRII (90,91); increased concentrations
of TNF-a independent of
atherosclerosis (91); elevated concentrations of IL-6 and CRP predicted
disability onset (92); increased concentrations of CRP and IL-6 were
associated with mortality (93); increased CRP concentrations in the elderly
was associated with development of diabetes mellitus (94); after challenge
with endotoxin aging was associated with more rapid increase in CRP and
prolonged inflammatory response and fever compared to younger individuals
(95); high levels of TNF-a were associated with high prevalence of atherosclerosis in 81-year-old
individuals (96); elevated concentrations of TNF-a predicted mortality in centenarians (97);
elevations in TNF-a and IL-6 were associated with mortality in 80-year-old people (98). |
|
Fever |
In periphery and brain increased concentrations of IL-1a, 1b, TNF-a and IL-6 (99); post-myocardial infarction patients with prolonged
fever had increased inflammatory activity (100). |
|
Gastrointestinal Disorders |
|
|
Crohn’s Disease |
High Th1 cell activity and increased proinflammatory state (101). |
|
Peptic Ulcer |
High ulcerogenic potential of Helicobacter pylori is linked, in part,
to increased activity of IL-8 and TNF-a (102); Helicobacter pylori and NSAIDs cause ulcer
recurrence through production of IL-1 and TNF-a by macrophages accumulated at the ulcer scar (103) |
|
Liver Disease |
Individuals afflicted with non-alcoholic steatohepatitis have
increased TNF-a levels compared to healthy persons and plays a role in pathogenesis
of disease (104); plasma levels of IL-18 and IL-18 binding protein are
elevated in patients with chronic liver disease and correlate with severity
of disease (105). |
|
Neurological Diseases |
|
|
Alzheimer’s Disease |
Neuroinflammation due to inflammatory mediator overexpression is
associated with behavioral disturbances (106); increased IL-1 expression in
Alzheimer brain is directly related to plaque formation and progression and
neuronal overexpression of acetylcholinesterase (107); TNF-a, IL-1b and IL-6 overexpression stimulated production of
amyloid-b which is crucial for
neurodegeneration in Alzheimer’s patients (108,109). |
|
Cerebral ischemia |
Increased IL-1, TNF-a, TNF-b and IL-6 concentrations in patients that have experienced acute
ischaemic brain injury (110,111); elevated IL-6 and TNF-b serum levels in acute stroke patients regardless of
subtype (110); increased expression of TNF-a, IL-1 and ICAM-1 (111). |
|
Down’s Syndrome |
Overexpression of IL-1 in middle-aged individuals that have
concurrent Alzheimer-type changes and in young and fetal Down’s patients
(109). |
|
Multiple Sclerosis |
Elevated TNF-a concentrations in serum and cerebral spinal fluid (112,113); brain
endothelium and astrocytes increased expression of ICAM-1 (114); increased
concentrations of LFA-1, ICAM-1, FA-3 adhesion molecules and chemokines
MCP-1, -2, -3, IP-10, GRO-a, RANTES, MIP-1a and -1b (110,115,116). |
|
Nutritional Disease |
Individuals undergoing long-term home parenteral nutrition without
clinical evidence of infection had increased sTNF-RII and IL-6 concentrations
indicating long-term home care total parenteral nutrition can be associated
with persistent low-grade inflammatory state (117); increased TFN-a concentrations in congestive heart failure patients
correlated independently with wasting (118-120); increased serum TNF-a levels in patients with gastrointestinal cancer
correlated with severity of weight loss (121). |
|
Obesity |
Elevated plasma CRP concentrations (122); increased concentrations of
TNF-a and soluble receptors
in overweight individuals associated with insulin resistance (123,124);
plasma TNF-a concentrations decreased with weight loss in obese individuals
(125); elevated IL-6 concentrations decreased in serum and subcutaneous
tissue of obese women after weight loss (126); elevated CRP, TNF-a and IL-6 concentrations have been linked to insulin resistance and
endothelial dysfunction with obesity and cardiovascular disease (127);
alterations in TNF-a gene locus involved with pathogenesis of obesity and
obesity-associated hypertension (128); TNF-a system was associated with altered plasma leptin
concentrations in obese individuals (129); increased IL-8 concentrations in
obese individuals and related to fat mass and TNF-a system (130); adipose tissue releases mediators
that influence body weight and inflammatory state (131); adipose tissue and
development of inflammatory state contributing to obesity associated
vasculopathy and cardiovascular disease (132). |
|
Diabetes |
Th-1 and Th-2 cells and their respective mediators participate and
cooperate in inducing and sustaining pancreatic islet cell β-cell
destruction in insulin dependent diabetes (133); inflammation important
factor in pathogenesis of diabetes and metabolic disorders in women (134);
increased CRP levels suggested to predict development of type 2 diabetes
(135); obesity and diabetes inflammatory states in which mediators of
inflammation contribute to insulin resistance (136). |
|
Pain |
Soluble TNF-a and IL-1 receptor antagonist administered intrathecally were
additive in reducing mechanical allodynia (137); IL-6 pathway is associated
with altered pain perception (138); hyperalgesia induced by TNF-a via stimulating release of IL-1 (139); hyperalgesia
induced by peripheral inflammation is associated with IL-1 overexpression
(140); spinal cord glia and glially derived proinflammatory cytokines
suggested to be powerful modulators of pain (141); interleukin-1b mediated induction of cyclooxygenase-2 in neurons
of the central nervous system contributes to inflammatory pain
hypersensitivity (142); bradykinin B2 receptors are suggested to
be involved with the acute phase of the inflammatory and pain response (143);
TNF-a expression is
suggested to be upregulated in Schwann cells influencing central pain
processing in painful neuropathies (144). |
|
Pancreatitis |
Matrix metalloprotinase-1, tissue inhibitor of metalloprotinase-1 and
TNF-a levels were higher in
non-survivors than survivors of acute pancreatitis (145); inflammatory
mediators TNF-a, Il-1b, IL-6, IL-8, PAF, IL-10, C5a, ICAM-1 and substance
P have a critical role in progression of acute pancreatitis (146). |
|
Parasitic Infections |
Increased TNF-a concentrations in patients with Plasmodium falciparum malaria is
associated with pathogenesis of disease (147). |
|
Psychiatric Disorders |
|
|
Delerium |
Increased concentrations of interferons and interleukins during
stress, rapid growth, inflammation, tumor, trauma and infection and
administration of interferons and interleukins are reported to be associated
with delerium (148). |
|
Dementia |
Increased IFN-a and decreased TGFb-1 were related to progression of AIDS dementia complex and has been
correlated with excessive neurocognitive dysfunction (149,150). |
|
Depression |
Increased expression of IL-1b, IL-6 and |
|
Dysthymia |
Increased production of IL-1b (153). |
|
Obsessive Compulsive Disorder |
Decreased plasma concentrations of IL-1b and TNF-a (154); decreased TNF-a but increased cortisol concentrations (155). |
|
Schizophrenia |
Increased concentrations of IL-6 and TNF-a (156-158); increased IL-1b polymorphism (159); drug-naïve schizophernic
patients had increased IL-2 and IFN-g production compared to controls (160). |
|
Sleep disorders |
TNF-a and IL-6 suggested to play an important role in mediating sleepiness
and fatigue in disorders of excessive daytime sleepiness (161); systemic
inflammatory response and reduced plasma availability of tryptophan was
related to primary sleep disorders and major depression (162). |
|
Stress |
Psychological stress is associated with increased production of TNF-a, IL-1, IL-1ra, IFN-g and lower production of IL-4 and IL-10 (163);
increased expression of neutrophils, monocytes, CD8+, CD2+CD26+
and CD2+HLA-DR+ T cells and CD19+ B cells
(164); post traumatic stress disorder was associated with increased IL-6
signaling (165). |
|
Rheumatoid Arthritis |
Increased concentrations of TNF-a as a central proinflammatory mediator (16,17)
increased concentrations of IL-1, IL-6, TNF-a, GM-CSF, and chemokines IL-8, RANTES, GRO-a, MIP-1a, MIP-1b, MCP-1 (16,17,166,167). |
|
Sepsis |
Systemic inflammatory response syndrome due to pro-inflammatory
mediator excess is associated with severe inflammatory responses then excessive
anti-inflammatory responses possibly leading to increased susceptibility to
infection (168-170); septic shock is caused at least in part by excessive or
dysregulated host inflammatory responses (171). |
|
Thyrotoxicosis |
Increased concentrations of IL-6 and IL-8 in patients afflicted with
thyrotoxicosis with levels decreasing as patients become euthyroid on
antithyroid treatment (172). |
|
Transplantation |
|
|
Heart |
Increased concentrations of TNF-a and IL-6 in myocardium of malfunctioning donor
hearts (173); association between increased IL-6 receptor concentrations and
acute cardiac allograft dysfunction in the early perioperative period (174);
pathological link between hypertension and increased NO production, decreased
asymmetric dimethylarginine levels, and TNF-a activation (175); lipid mediators of inflammation
implicated in allograft rejection (176). |
|
Liver |
TNF-a polymorphism associated with increased production associated with
acute hepatocellular rejection (177); Th2 cytokine production is associated
with improved graft acceptance in infants after liver transplantation (46);
increased IL-8, IL-10 and TNF-a in during first post-operative week patients
developed serious complications within the first month after surgery (178). |
|
Lung |
Increased expression of IL-1ra was associated with development of
bronchiolitis obliterans syndrome a major limitation to survival (179). |
|
Kidney |
High IFN-g production influenced acute rejection of kidney transplant (180). |
|
Tuberculosis |
Serum concentrations of sTNFRI and II and IL-1ra may serve as markers
of disease activity (181); tuberculosis osteomyelitis is associated with
elevated IL-6 concentrations (182). |
|
Injury |
|
|
Burn |
Increased IL-1 and IL-1b concentrations in burn patients and IL-6
concentrations were greater in those that died of their burn injuries
compared to survivors (183). |
|
Trauma |
Peak levels of sICAM-1, sVCAM-1 correlated with disseminated
intravascular coagulation and sustained inflammation caused by neutrophil
endothelium interaction gave rise to multiple organ dysfunction syndrome resulting in poor patient outcome (184). |
|
|
|
Table 2: Increased
inflammatory mediator expression and pharmacological response
|
Disease |
Pharmacological
Response |
|
Acquired
Immunodeficiency Syndrome (AIDS) |
African patients with AIDS exhibit
greater TNF-a concentrations that may be
responsible for reduced response to pharmacotherapy for tuberculosis HIV-1
load (219); persistent TNF-a activation is involved in
highly active antiretroviral therapy failure (220); decrease in T-cells producing
TNF-a and IL-4 are suggested to be
early predictors of early response to and early failure of highly active
antiretroviral therapy (221). |
|
Atopic Disease |
Glucocortocosteroid non-responsive bronchial
hyper-responsiveness in mild asthma is associated with overproduction of IL-5
by lymphocytes (222); reduction in serum IL-5 was associated with resolution
of atopic dermatitis (223); IL-6 overexpression is involved with pathogenesis
of severe acute urticaria that is resistant to antihistamine treatment (224). |
|
Cancer |
Induction of doxorubicin resistance was
associated with increased intracellular levels of TNF-a (225); increased concentrations of TGF-b are associated with resistance of prostate carcinoma
to cytotoxic cancer therapy (226); resistance to tamoxifen in human breast
carcinoma is linked to overexpression of TGF-b2 (227); increased IL-6
concentrations was related to insulin resistance in cancer patients (228);
effectiveness of cyclophosphamide is suggested to be due to a pattern shift
of cytokines from Th2 to Th1 around the tumor lesion (229). |
|
Cardiovascular Disorders |
|
|
Acute myocardial infarction |
Increase mortality after acute myocardial
infarction is associated with overexpression of CRP independent of coronary
revascularization procedures performed and medical therapy (214). |
|
Arrhythmia |
Reduced PR interval response to verapamil in
rheumatoid arthritic patients (230). |
|
Unstable angina |
Greater CRP, fibrinogen and erythrocyte
sedimentation rate in patients with refractory angina as compared to those stabilized
(201); complicated hospital course linked with increased IL-1ra and IL-6
concentrations and an uneventful course when IL-1ra and IL-6 concentrations
decreased 48 hours post-admission (200). |
|
Hyperlipidemia |
Reduced CRP levels in survivors of myocardial
infarction when under therapy with hypolipidemic drug pravastatin which was
independent of the magnitude of lipid alterations (231); statins are
suggested to modulate immune responses by inhibiting induction of MHC-II
expression by IFN-g thus acting as suppressors
of MHC-II mediated T-cell activation (232); lowering CRP with statins
associated with better clinical outcome (207). |
|
Diabetes |
Overexpression of TNF-a in human muscle tissue was associated with insulin
resistance (233). |
|
Elderly |
Reduced sensitivity of L-type calcium channels
(234,235); hypertensive patients that received a b-blocker had no benefit of reduced coronary events
(236); b-blocker not efficacious as
first line therapy in hypertensive elderly patients (237). |
|
Infectious Diseases |
High levels of inflammatory cytokines are
associated with poor clinical response to steroid treatment also recurrent
episodes in leprosy patients (238); reduced myocardial responsiveness in
septic shock patients suggested to be due to reduced b-adrenergic receptor function (239); increased plasma
TNF-a plasma levels were
associated with patient deterioration early in treatment of severe
tuberculosis (240). |
|
Obesity |
Reduced sensitivity to verapamil in overweight
individuals (241). |
|
Pain |
Chemotactic activities of m- and d-opioid receptors are
desensitized following activation of chemokine receptors suggesting that
activation of proinflammatory chemokine receptor downregulates analgesic
function of opioid receptors enhancing pain perceptions (242). |
|
Psychiatric conditions |
|
|
Depression |
Increased IL-6 concentrations were associated
with treatment failure (243). |
|
Schizophrenia |
Increased
IL-6 concentrations were associated with refractory disease (244); successful
antipsychotic therapy was associated with reduction in inflammatory state and
normalization of immune responses (245,246). |
|
Rheumatoid Arthritis |
Restoring Th1/Th2 cell balance reverses
treatment-refractory arthritis (247); resistance to disease-modifying drugs
was associated with increased Th1 cells expressing p-glycoproteins (248). |
|
|
|
1.
Delves PJ, Riott IM. The immune system. N Eng J Med 343:37-49, 108-117,
2000.
2. Haynes BF,
3.
Le Moine A,
Goldman M, Abramowicz D. Multiple pathways to allograft rejection.
Transplantation 73:1373-1381, 2002.
4. Roayaie S, Sheiner
PA, Emre S, Guy S, Schwartz ME, Boros P, Miller CM. Cytokine profiles in early
rejection following OKT3 treatment in liver transplant patients. Mediators
Inflamm 9:141-146, 2000.
5. Sallusto F,
Lanzavecchia A, Mackay CR. Chemokines and chemokine receptors in T-cell priming
and Th1/Th2-mediated responses. Immunol Today 19:568-574, 1998.
6.
Morel PA, Oriss TB.
Crossregulation between Th1 and Th2 cells. Crit Rev Immunol 18:275-303, 1998.
7.
O’Shea JJ, Ma A,
Lipsky P. Cytokines in autoimmunity. Nat Rev Immunol 2:37-45, 2002.
8.
Singh VK, Mehrotra
S, Agarwal SS. The paradigm of Th1 and Th2 cytokines: its relevance to
autoimmunity and allergy. Immunol Res 20:147-161, 1999.
10.
O’Gara A.
Cytokines induce the development of functionally heterogeneous T helper cell
subsets. Immunity 8:275-283, 1998.
11.
12.
Kulmatycki KM,
Jamali F. Therapeutic relevance of altered cytokine expression. Cytokine.
14:1-10, 2001.
13.
http://www.academicpress.com/cytokinereference
14.
Ozaki K, Leonard
WJ. Cytokine and cytokine receptor pleiotropy and redundancy. J Biol Chem 277:29355-29358,
2002.
15.
Sanchez-Cuenca J,
Martin JC, Pellicer A, Simon C. Cytokine pleiotropy and redundancy – gp 130
cytokines in human implantation. Immunol Today 20:57-59, 1999.
16.
Feldmann M, Brennan FM, Maini RN. Role of cytokines in rheumatoid arthritis. Ann Rev
Immunol. 14:397-440, 1996.
17.
Charles P,
Elliott MJ, Davis D, Potter A, Kalden JR, Antoni C,
Breedveld FC, Smolen JS, Ebrel G, deWoody K, Feldmann M, Maini RN. Regulation
of cytokines, cytokine inhibitors, and acute-phase proteins following anti-TNF-a therapy in rheumatoid arthritis. J Immunol
163:1521-1528, 1999.
18. Comini L,
Bachetti T, Agnoletti L, Gaia G, Curello S, Milanesi B, Volterrani M,
Parrinello G, Ceconi PC, Giordano A, Corti A, Ferrari R. Induction of
functional inducible nitric oxide synthase in monocytes of patients with
congestive heart failure. Link with tumor necrosis factor-a. Eur Heart J 20:1503-1513, 1999.
19.
Bellingan G.
Inflammatory cell activation in sepsis. Br Med Bull 55:1:12-29, 1999.
20.
Lauta VM. A
review of the cytokine network in multiple myeloma: diagnostic, prognostic, and
therapeutic implications. Cancer 97:2440-2452, 2003.
21.
Locati M, Murphy
PM. Chemokines and chemokine receptors: biology and clinical relevance in
inflammation and AIDS. Annu Rev Med 50:425-440, 1999.
22.
Locati M, Otero
K, Schioppa T, Signorelli P, Perrier P, Baviera S, Sozzani S, Mantovani A. The
chemokine system: tuning and shaping by regulation of receptor expression and
coupling in polarized responses. Allergy 57:972-982, 2002.
23.
Lukacs NW,
Hogaboam C, Campbell E, Kunkel SL. Chemokines: function, regulation and
alteration of inflammatory responses. Chem Immunol 72:102-120, 1999.
24.
Luster AD. The
role of chemokines in linking innate and adaptive immunity. Curr Opin Immunol
14:129-135, 2002.
25.
Ward PA,
26.
Coleman JW.
Nitric oxide in immunity and inflammation. Int Immunopharmacol 1:1397-1406,
2001.
27.
Coleman JW.
Nitric oxide: a regulator of mast cell activation and mast cell-mediated
inflammation. Clin Exp Immunol 129:4-10, 2002.
28.
Ignarro LJ,
Cirino G, Casini A, Napoli C. Nitric oxide as a signaling molecule in the
vascular system: an overview. J Cardiovasc Pharmacol 34:879-886, 1999.
29.
Kalra DK,
Ramchandani M, Zhu X, Lawrie G, Reardon MJ, Mann DL, Zoghbi WA, Nagueh SF.
Relation of tissue doppler-derived myocardial velocities to serum levels and
myocardial gene expression of tumor necrosis factor-alpha and inducible nitric
oxide synthase in patients with ischemic cardiomyopathy having coronary artery
bypass grafting. Am J Cadiol 90:708-712, 2002.
30.
Alderton WK,
Cooper CE, Knowles RG. Nitric oxide synthases: structure, function and
inhibition. Biochem J 357:593-615, 2001.
31.
Tanus-Santos JE,
Desai M, Deak LR, Pezzullo JC, Abernethy DR, Flockhart DA, Freedman JE. Effects
of endothelial nitric oxide synthase gene polymorphisms on platelet function,
nitric oxide release, and interactions with estradiol. Pharmacogenetics
12:407-413, 2002.
32.
Groeneveld PHP,
Kwappenberg KMC, Langermans JAM, Nibbering PH, Curtis L. Relation between pro-
and antiinflammatory cytokines and the production of nitric oxide (NO) in
severe sepsis. Cytokine 9:138-142, 1997.
33.
http://www.copewithcytokines.de/cope.cgi.html
34.
Vicenzi E, Biswas
P, Mengozzi M, Poli G. Role of pro-inflammatory cytokines and b-chemokines in controlling HIV replication. J Leukoc
Biol 62:34-40, 1997.
35.
Riffo-Vasquez Y,
Spina D. Role of cytokines and chemokines in bronchial hyperresponsiveness and
airway inflammation. Pharmacol Ther 94:185-211, 2002.
36.
Libby P, Ridker
PM, Maseri A. Inflammation and atherosclerosis. Circulation 105:1135-1143,
2002.
37.
Coussens LM, Werb
Z. Inflammation and cancer. Nature 420:860-867, 2002.
38.
Mann DL.
Inflammatory mediators and the failing heart past, present, and the foreseeable
future. Circ Res 91:988-998, 2002.
39.
Kofler S, Nickel
T, Weis M. Role of cytokines in cardiovascular diseases: a focus on endothelial
responses to inflammation. Clin Sci 108:205-213, 2005.
40.
41.
Freeman DJ,
Norrie J, Caslake MJ, Gaw A, Ford I, Lowe GDO, O’Reilly DS, Packard CJ, Sattar
N. West of Scotland Coronary Prevention Study. C-reactive protein is an
independent predictor of risk for the development of diabetes in the West of
Scotland Coronary Prevention Study. Diabetes 51:1596-1600, 2002.
42.
Dandona P, Aljada
A, Bandyopadhyay A. Inflammation: the link between insulin resistance, obesity
and diabetes. Trend Immunol 25:4-7, 2004.
43.
Dalgleish AG,
O’Byrne KJ. Chronic immune activation and inflammation as the cause of malignancy.
Br J Cancer 85:473-483, 2001.
44.
Maes M, Meltzer
HY, Bosmans E, Bergmans R, Vandoolaeghe E, Ranjan R, Desnyder R. Increased
plasma concentrations of interleukin-6, soluble interleukin-6, soluble
interleukin-2 and transferrin receptor in major depression. J Affect Disord
34:301-309, 1995.
46.
Ganschow R,
Broering DC, Nolkemper D, Albani J, Kemper MJ, Rogiers X, Burdelski M. Th2
cytokine profile in infants predisposes to improved graft acceptance after
liver transplantation. Transplantation 72:929-934, 2001.
47.
Emilie D,
Galanaud P. Cytokines and chemokines in HIV infection: implications for
therapy. Intern Rev Immunol 16:705-726, 1998.
48.
Lore K,
Sonnerborg A, Olsson J, Patterson BK, Fehniger TE, Perbeck L, Andersson J.
HIV-1 exposed dentritic cells show increased pro-inflammatory cytokine
production but reduced IL-1ra following lipopolysaccharide stimulation. AIDS
13:2013-2021, 1999.
49.
Lee B, Montaner
LJ. Chemokine immunobiology in HIV-1 pathogenesis. J Leukoc Biol 65:552-565,
1999.
50.
Breen EC. Pro-
and antiinflammatory cytokines in human immunodeficiency virus infection and
acquired immunodeficiency syndrome. Pharmacology and Therapeutics 95:295-304,
2002.
51.
Kulander L,
Pauksens K, Venge P. Soluble adhesion molecules, cytokines and cellular markers
in serum in patients with acute infections.
Scand J Infect Dis 33:290-300, 2001.
52.
de Vires JE, Carballido JM, Aversa G. Receptors and
cytokines involved in allergic TH2 cell responses. J Allergy Clin
Immunol 103(5 pt 2):S492-S496, 1999.
53.
Bellanti JA.
Cytokines and allergic diseases: clinical aspects. Allergy Asthma Proc
19:337-341, 1998.
54.
Romagnani S.
T-cell subsets (Th1 versus Th2). Ann Allergy Asthma Immunol 85:9-21, 2000.
55.
McDyer JF, Wu
C-Y, Seder RA. The regulation of IL-12: its role in infectious, autoimmune, and
allergic diseases. J Allergy Clin Immunol 102:11-15, 1998.
56.
Silvestri M,
Sabatini F, Defilippi AC, Ghiro L, Baraldi E, Rossi GA. A marker of asthma
inflammation: orally exhaled nitric oxide. ACI International 15:37-43, 2003.
57.
Lalani T, Simmons
RK, Ahmed AR. Biology of IL-5 in health and disease. Ann Allergy Asthma Immunol 82:317-333, 1999.
58.
Hamzaoui K,
Hamzaoui A, Guemira F, Bessioud M, Hamza M, Ayed K. Cytokine profile in BehVet’s disease patients. Scan J Rheumatol 31:205-210, 2002.
59.
Shariat SF,
Andrews B, Kattan MW, Kim J, Wheeler TM, Slawin KM, Plasma levels of
interleukin-6 and its soluble receptor are associated with prostate cancer
progression and metastasis. Urology 58:1008-1015, 2001.
60.
Salem M, Elbaz O,
Zahran M, Elbaz A, Elgamal S, Eteba S and Mahmoud LA. Malignancy:
identification of predictors of disease status and progression in patients with
myeloma (MM). Hematol 5:1:41-45, 2000.
61.
Numasaki M,
Fukushi J-I, Ono M, Narula SK, Zavodny PJ, Kudo T, Robbins PD, Tahara H, Lotze
MT. Interleukin-17 promotes angiogenesis and tumor growth. Blood 101:2620-2627,
2003.
62.
Miyao Y, Yasue H,
Ogawa H, Misumi I, Masuda T, Sakamoto T, Morita E. Elevated plasma
interleukin-6 levels in patients with acute myocardial infarction. Am Heart J
126:1299-1304, 1993.
63.
Lagrand WK,
Niessen HWM, Wolbink GJ, Jaspars LH, Visser CA, Verheugt FWA, Meijer CJLM, Hack
CE. C-reactive protein colocalizes with complement in human hearts during acute
myocardial infarction. Circulation 95:97-103, 1997.
64.
Guillen I, Blanes M, Gomez-Lechon MJ, Castell JV. Cytokine signaling during myocardial infarction:
sequential appearance of IL-1b and IL-6. Am J Physiol 269:R229-R235, 1995.
65.
Irwin MW, Mak S,
Mann DL, Qu R, Penninger JM, Yan A, Dawood F, Wen H, Shou Z, Liu P. Tissue
expression and immunolocalization of tumor necrosis factor-a in postinfarction dysfunctional myocardium.
Circulation 99:1492-1498, 1999.
66.
Rossi E, Biasucci
LM, Citterio F, Pelliccioni S, Monaco C, Ginnetti F, Angiolillo DJ, Grieco G,
Liuzzo G, Maseri A. Risk of myocardial infarction and angina in patients with
severe peripheral vascular disease predictive role of C-reactive protein.
Circulation 105:800-803, 2002.
67.
Zairis MN,
Manousakis SJ, Stefanidis AS, Papadki OA, Andrikopoulos GK, Olympios CD,
Hadjissavas JJ, Argyrakis SK, Foussas SG. C-reative protein levels on admission
are associated with response to thrombolysis and prognosis after ST-segment
elevation acute myocardial infarction. Am Heart J 144:782-789, 2002.
68.
Rifai N, Ridker
PM. Inflammatory markers and coronary heart disease. Curr Opin Lipidol
13:383-389, 2002.
69.
Cockerill GW,
Saklatvala J, Ridley SH, Yarwood H, Miller NE, Oral B, Nithyanathan S, Taylor
G, Haskard DO. High-density lipoproteins differentially modulate
cytokine-induced expression of E-selectin and cyclooxygenase-2. Arterioscler Thromb
Vasc Biol 19:910-917, 1999.
70.
Porsch-Oezcueruemez
M, Kunz D, Kloer HU, Luley C. Evaluation of serum levels of solubilized
adhesion molecules and cytokine receptors in coronary heart disease. J Am Coll
Cardiol 34:195-2001, 1999.
71.
Calebrsi L,
Franceschini G, Sirtori CR, Plama A, De Sarsella M, Ferrante P, Taramelli D.
Inhibition of VCAM-1 expression in endothelial cells by reconstituted high
density lipoproteins. Biochem Biophys Res Commun 238:61-65, 1996.
72.
Behrendt D, Ganz
P. Endothelial function: from vascular biology to clinical applications. Am J Cardiol 90(suppl) 40L-48L, 2002.
73.
Koenig W, Sund M,
Fröhlich M, Fisher HG, Löwel H, Döring A, Hutchinson WL, Pepys MB. C-reactive
protein, a sensitive marker of inflammation, predicts future risk of coronary
heart disease in initially healthy middle-aged men. Circulation 99:237-242,
1999.
74.
Mazer SP, Rabbani
LE. Evidence for C-reactive protein’s role in (CRP) vascular disease:
atherothrombosis, immuno-regulation and CRP. J Thrombosis and Thrombolysis
17:95-105, 2004.
75.
Mann DL.
Stress-activated cytokines and the heart: from adaption to maladaption. Annu
Rev Physiol 65:81-101, 2003
76.
Torre-Amione G, Vooletich MT, Farmer JA. Role of tumor necrosis factor-a in the progression of heart failure. Drugs
59:4:745-751, 2000.
77.
Tsutamoto T,
Hisanaga T, Atsuyuki W, Maeda K, Ohnishi M, Fukai D, Mabuchi N, Sawaki M,
Kinoshita M. Interleukin-6 spillover in the peripheral circulation increases
with the severity of heart failure, and the high plasma level of interleukin-6
is an important prognostic predictor in patients with congestive heart failure.
J Am Coll Cardiol 31:391-398, 1998.
78.
Milani RV, Mehr
MR, Endres S, Eigler A, Cooper S, Lavie CJ Jr., Ventura HO. The clinical
relevence of circulating tumor necrosis factor-a in acute decompensated chronic heart failure without
cachexia. Chest 110:992-995, 1996
79.
Peeters ACTM,
Netea MG, Janssen MCH, Kullberg BJ, Van der Meer JWM, Thien T. Pro-inflammatory
cytokines in patients with essential hypertension. Eur J Clin Invest 31-36,
2001
80.
Ouvina SM, La
Greca RD, Zanaro NL, Palmer L, Sassetti B. Endothelial dysfunction, nitric
oxide and platelet activation in hypertensive and diabetic type II patients.
Thrombosis Research 102:107-114, 2001.
81.
Sung KC, Suh JY,
Kim BS, Kang JH, Kim H, Lee MH, Park JR, Kim SW. High sensitivity C-reactive
protein as an independent risk factor for essential hypertension. AHJ
16:429-433, 2003.
82.
Waehre T,
Halvorsen B, Damas JK, Yndestad A, Brosstad F, Gullestad L, Kjekshus J, Froland
SS, Aukhurst P. Inflammatory imbalance between IL-10 and TNFa in unstable angina potential plaque stabilizing
effects of IL-10. Eur J Clin Invest 32:803-810, 2002.
83.
Liuzzo G,
Biasucci LM, Gallimore JR, Caligiuri G, Buffon A, Rebuzzi AG, Pepys MB, Maseri
A. Enhanced inflammatory response in patients with preinfarction unstable
angina. J Am Coll Cardiol 34:1696-1703, 1999.
84.
Ikonomidis I,
Andreotti F, Economou E, Stefanadis C, Toutouzas P, Nihoyannopoulos P.
Increased proinflammatory cytokines in patients with chronic stable angina and
their reduction by aspirin. Circulation 100:793-798, 1999.
85.
Buffon A, Liuzzo
G, Biasucci LM, Pasqualetti P, Ramazzotti V, Rebuzzi AG, Crea F and Maseri A.
Preprocedural serum levels of C-reactive protein predict early complications
and late restenosis after coronary angioplasty. J Am Coll Cardiol 34:1512-1521,
1999.
86.
Anguera I,
Miranda-Guardiola F, Bosch X, Filella X, Sitges M, Marin JL, Betriu A, Sanz G.
Elevation of serum levels of the antiinflammatory cytokine interleukin-10 and
decreased risk of coronary events in patients with unstable angina. Am Heart J 144:811-817, 2002.
87.
Lindmark E,
Diderholm E, Wallentin L, Sieghdahn A. Relationship between interleukin 6 and
mortality in patients with unstable coronary artery disease. JAMA
286:2107-2113, 2001.
88.
Vila N, Castillo
J, Davalos A, Esteve A, Planas A and Chamorro A. Levels of antiinflammatory
cytokines and neurological worsening in acute aschemic stroke. Stroke 34:671-675,
2003
89.
Mo R, Chen J, Han
Y, Bueno-Cannizares C, Misek DE, Lescure PA, Hanash S, Yung RL. T cell
chemokine receptor expression in aging. J Immunol 170:895-904, 2003.
90.
Liao Z, Tu JH,
Small CB, Schnipper SM, Rosenstreich DL. Increased urine interleukin-1 levels
in aging. Geronotology 39:19-27, 1993.
91.
Bruunsgaard H,
Anderson-Ranberg K, Juene B, Pedersen AN, Skinhoj P, Pedersen BK. A high plasma
concentration of TNF-a is associated with dementia in centenarians. J Gerentol A Biol Sci Med
Sci 54A:M357-M364, 1999.
92.
Ferrucci L,
Harris TB, Guralnik JM, Tracy RP, Corti M-C, Cohen HJ, Penninx B, Pahor M,
Wallace R, Havlik RJ. Serum IL-6 level and the development of disability in
older persons. J Am Geriatr Soc 47:639-647, 1999.
93.
Harris TB,
Ferrucci L, Tracy RP, Corti MC, Wacholder S, Ettinger WH Jr., Heimovitz H,
Cohen HJ, Wallace R. Associations of elevated interleukin-6 and C-reactive
protein levels with mortality in the elderly. Am J Med
106:506-512, 1999.
94.
Barzilay JI,
Abraham L, Heckbert SR, Cushman M, Kuller LH, Resnick HE, Tracy RP. The
relation of markers of inflammation to the development of glucose disorders in
the elderly. Diabetes 50:2384-2389, 2001
95.
Krabbe SK,
Bruunsgaard H, Hansen CM, Moller K, Fonsmark L, Qvist J, Madsen PL, Kronborg G,
Andersen HO, Skinhoj P, Pedersen BK. Ageing is associated with a prolonged
fever response in human endotoxemia. Clinical and Diag Lab Immunol 8:333-338,
2001.
96.
Bruunsgaard H,
Skinhoj P, Pdeersen AN, Schroll M, Pedersen KB. Ageing, tumor necrosis
factor-alpha (TNF-a) and atherosclerosis. Clin Exp Immunol 121:255-260.
97.
Bruunsgaard H,
Andersen-Ramberg K, Hjelmborg JBV, Pedersen BK, Jeune B. Elevated levels of tumor
necrosis factor alpha and mortality in centenarians. Am
J Med 115:278-283, 2003.
98.
Bruunsgaard H,
Ladelund S, Pedersen AN, Schroll M, Jorgensen T. Predicting death from tumor
necrosis factor-alpha and interleukin-6 in 80-year old people. Clin Exp Immunol
132:24-31, 2003.
99.
Zetterstrom M,
100.
Gabriel AS, Ahnve
S, Wretlind B, Martinsson A. IL-6 and IL-1 receptor antagonist in stable angina
pectoris and relation of IL-6 to clinical findings in acute myocardial
infarction. J Intern Med 248:61-66, 2000.
101.
van Hogezand RA, Verspaget HW. The future role of anti-tumor necrosis factor-a products in the treatment of Crohn’s disease. Drugs
56:299-305, 1998.
102.
Lehmann FS,
Stalder GA. Hypothesis on the role of cytokines in peptic ulcer disease. Eur J
Clin Invest 28:511-519, 1998.
103.
Arakawa T,
Watanabe T, Fukuda T, Higuchi K, Fujiwara Y, Kobayashi K, Tarnawski. Ulcer recurrence
cytokines and inflammatory response-dependent process. Dig Dis Sci 43:61S-66S,
1998.
104.
Wigg AJ,
Roberts-Thomson IC, Dymock RB, McCarthy PJ, Grose RH, Cummins AG. The role of
small intestine bacterial overgrowth, intestinal permeability, endotoxaemia,
and tumor necrosis factor a in the pathogenesis of non-alcoholic steatohepatitis. Gut 48:206-211,
2001.
105.
Ludwiczek O,
Kaser A, Novick D, Dinarello CA, Rubinstein M, Vogel W, Tilg N. Plasma levels
of interleukin-18 and interleukin-18 binding protein are elevated in patients
with chronic liver disease. J Clin Immnol 22:331-337, 2002.
106.
Eikelenboom P,
Hoogendijk WJG, Jonker C, van Tilburg W. Immunological mechanisms and the
spectrum of psychiatric syndromes in Alzheimer’s disease. Journal of
Psychiatric Research 36:269-280, 2002.
107.
Mrak RE,
108.
Blasko I,
Grubeck-Loebenstein B. Role of immune system in the pathogenesis, prevention
and treatment of Alzheimer’s disease. Drugs
Aging 20:101-113, 2003.
109.
110.
Sharma BK, Kumar
K. Role of proinflammatory cytokines in cerebral ischemia: a review. Metab
Brain Dis 13:1-8, 1998.
111.
Pantoni L, Sarti
C, Inzitari D. Cytokines and cell adhesion molecules in cerebral ischemia.
Arterioscler Thromb Vasc Biol 18:503-513, 1998.
112.
Ledeen RW,
Chakraborty G. Cytokines signal transduction, and inflammatory demyelination:
review and hypothesis. Neurochemical Res 23:277-289, 1998.
113.
Lenercept
Multiple Sclerosis Study Group. TNF neutralization in MS results of a randomized,
placebo-controlled multicenter study. Neurology 53:457-465, 1999.
114.
Munoz-Fernandez
MA,
115.
116.
Horuk H, Ng HP.
Chemokine receptor antagonists. Med Res Rev 20:155-168, 2000.
117.
Ling PR,
Khaodhiar L, Bistrian BR, Keane-Ellison M, Thibault A, Tawa N. Inflammatory
mediators in patients receiving long-term home parenteral nutrition. Dig Dis
and Sci 46:11:2484-2489, 2001.
118.
Anker SD ,
Ponikowski P, Varney S, Chua TP, Clark AL, Webb-Peploe KM, Harrington D, Kox
WJ, Poole-Wilson PA, Coats AJS. Wasting as independent risk factor for
mortality in chronic heart failure. Lancet
349:1050-1053, 1997.
119.
Anker SD,
Ponikowski PP, Clark AL, Leyva F, Rauchhaus M, Kemp M, Teixeira MM, Hellewell
PG, Hooper J, Poole-Wilson PA, Coats AJS. Cytokines and neurohormones relating
to body composition alterations in the wasting syndrome of chronic heart
failure. Eur Heart J 20:683-693, 1999.
120.
121.
Bossola M,
Muscaritoli M, Bellantone R, Pacelli F, Cascino A, Sgadari A, Battaglia F,
Piccioni E, Scambia G, Doglietto GB, Fanelli FR. Serum tumor necrosis factor-a levels in cancer patients are discontinuous and
correlate with weight loss. Eur J Clin Invest 30:12:1107-1112, 2000.
122.
Visser M, Bouter
LM, McQuillan GM, Wener MH, Harris TB. Elevated C-reactive protein levels in
overweight and obese adults. JAMA 282:2131-2135, 1999.
123.
Hauner H, Bender
M, Haastert B, Hube F. Plasma concentrations of soluble TNF-alpha receptors in
obese subjects. International Journal of Obesity 22:1239-1243, 1998.
124.
Xu H, Uysal KT,
Becherer JD, Arner P, Hotamisligil GS. Altered tumor necrosis factor-a (TNF-a) processing in adipocytes and increased expression of transmembrane
TNF-a in obesity. Diabetes
51:1876-1883, 2002.
125.
Dandona P,
Weinstock R, Thusu K, Abdel-Rahman E, Aljada A, Wadden T.
Tumor necrosis factor-a in sera of obese patients: fall with weight
loss. J Clin Endocrinol Metab 83:2907-2910, 1998.
126.
Bastard JP,
Jardel C, Bruckert E, Blondy P, Capeau J, Laville M, Vidal H, Hainque B.
Elevated levels of interleukin-6 are reduced in serum and subcutaneous adipoise
tissue of obese women after weight loss. J Clin Endocrinol Metab 85:3338-3342,
2000.
127.
Yudkin JS,
Stehouwer CDA, Emeis JJ, Coppack SW. C-reactive protein in healthy subjects:
associations with obesity, insulin resistance, and endothelial dysfunction a
potential role for cytokines originating from adipose tissue? Arterioscler
Thromb Vasc Biol 19:972-978, 1999.
128.
Pausova Z,
Deslauriers B, Gaudet D, Trembly J, Kotchen TA, Larochelle P, Cowley AW, Hamet
P. Role of tumor necrosis factor-a gene locus in obesity and obesity-associated
hypertension in French Canadians. Hypertension 36:14-19, 2000.
129.
Corica F, Allegra
A, Corsonello A, Buemi M, Calapai G, Ruello A, Mauro VN, Ceruso D. Relationship
between plasma leptin levels and the tumor necrosis factor-a system in obese subjects. Int J of Obesity 23:355-360,
1999.
130.
Straczkowski M,
Dzienis-Straczkowska S, Stepien A, Kowalska I, Szelachowska M, Kinalska I.
Plasma interleukin-8 concentrations are increased in obese subjects and related
to fat mass and tumor necosis factor-a system. J Clin Endocrinol Metab 87:4602-4606, 2002.
131.
Lau DCW, Dhillon
B, Yan H, Szmitko PE, Verma S. Adipokines: molecular links between obesity and
atherosclerosis. Am J Physiol Heart Circ Physiol 288:H2031-H2041, 2005.
132.
Berg AH,
133.
134.
Han TS, Sattar N,
Williams K. Prospective study of C-reactive protein in relation to the
development of diabetes and metabolic syndrome in the
135.
Sjöholm A,
Nyström T. Endothelial inflammation in insulin resistance. Lancet 365:610-612,
2005.
136.
Dandona P, Aljada
A, Bandyopadhyay A. Inflammation: the link between insulin resistance, obesity
and diabetes. Trends Immunol 25:4-7, 2004.
137.
Rutowski MD,
DeLeo JA. The role of cytokines in the initiation and maintenance of chronic
pain. Drug News Perspect 15:626-632, 2002.
138.
De Jongh RF, Vissers KC, Meert TF, Booij LHDJ, De Deyne
CS, Heylen RJ. The role of interleukin-6
in nociceptin and pain. Anesth Analg 96:1096-1103, 2003.
139.
Watkins LR, Maier SF, Goehler LE. Immune activation: the role of pro-inflammatory cytokines
in inflammation, illness responses and pathological pain states. Pain
63:289-302, 1995.
140.
Watkins LR, Maier
SF. Implications of immune-to-brain communication for sickness and pain. Proc
Natl Acad Sci 96:7710-7713, 1999.
141.
Wieseler-Frank J, Maier SF, Watkins LR. Immune-to-brain communication dynamically modulates
pain: physiological and pathological consequences. Brain Behav Immun
19:104-111, 2005.
142.
Samad TA, Moore
KA, Sapirstein A, Billet S, Allchrone A, Poole S, Bonventre JV, Woolf CJ.
Interleukin-1b-mediated induction of Cox-2 in the CNS contributes to inflammatory
pain hypersensitivity. Nature 410:471-475, 2001.
143.
Couture R,
Harrison M, Vianna RM, Cloutier F. Kinin receptors in pain and inflammation.
Eur J of Pharmacol 429:161-176, 2001.
144.
Empl M, Renaud S, Erne B, Fuhr P, Straube A,
Schaeren-Wiemers N, Steck AJ. TNF-alpha
expression in painful and nonpainful neuropathies. Neurology 56:1371-1377,
2001.
145.
Nakae H, Endo S,
Inoue Y, Fujino Y, Wakabayashi G, Inada K, Sato S. Matrix metalloproteinase-1
and cytokines in patients with acute pancreatitis. Pancreas 26:2:134-138, 2003.
146.
Bhatia M, Brady
M, Shokuhi S, Christmas S, Neoptolemos JP, Slavin J. Inflammatory mediators in
acute pancreatitis. J Pathol 190:117-125, 2000.
147.
Odeh M. The role
of tumor necrosis factor-a in the pathogenensis of complicated Falciparum malaria. Cytokine 14:1:11-18, 2001.
148.
Trzepacz PT.
Delerium advances in diagnosis, pathophysiology, and treatment. Psychiatr Clin
North Am 19:3:429-448, 1996.
149.
Perrella O,
Carrieri PB, Perrella A, Sbreglia C, Gorga F, Guarnaccia D, Tarantino G.
Transforming growth factor-beta 1 and interferon-alpha in the AIDS demetia
complex (ADC): possible relationship with cerebral viral load? Eur Cytokine
Netw 12:51-55, 2001.
150.
151.
Connor TJ,
Leonard. Depression, stress and immunological activation: the role of cytokines
in depressive disorders. Life Sci 62:583-606, 1998.
152.
Levine J, Barak
Y, Chengappa KNR, Rapoport A, Rebey M, Barak V. Cerebrospinal cytokine levels
in patients with acute depression. Neuropsychobiology 40:171-176, 1999.
153.
Anisman H,
Ravindran AV, Griffiths J, Merali Z. Interleukin-1b production in dysthymia before and after
pharmacotherapy. Biol Psychiatry 46:1649-1655, 1999.
154.
Brambilla F,
Perna G, Bellodi L, Arancio C, Bertani A, Perini G, Carraro C, Gava F. Plasma
interleukin-1b and tumor necrosis factor concentrations in obsessive-compulsive
disorders. Biol Psychiatry 42:976-981,
1997.
155.
Monteleone P,
Catapano F, Fabrazzo M, Tortorella A, Maj M. Decreased blood levels of tumor
necrosis factor-alpha in patients with obsessive-compulsive disorder.
Neuropsychobiology 37:182-185, 1998.
156.
Frommberger UH,
Bauer J, Haselbauer P, Fraulin A, Riemann D, Berger M. Interleukin-6-(IL-6)
plasma levels in depression and schizophrenia: comparison between the acute
state and after remission. Eur Arch Psychiatry Clin Neurosci 247:228-233, 1997
157.
Naudin J, Mege
JL, Azorin JM, Dassa D. Elevated circulating levels of IL-6 in schizophrenia.
Schizophr Res 20:269-273, 1996.
158.
Monteleone P,
Fabrazzo M, Tortorella A, Maj M. Plasma levels of interleukin-6 and tumor
necrosis factor alpha in chronic schizophrenia: effects of clozapine treatment.
Psychiatry Res 71:11-17, 1997.
159.
Katila H,
Hanninen K, Hurme M. Polymorphisms of the interleukin-1 gene complex in
schizophrenia. Mol Psychiatry 4:179-181, 1999.
160.
Cazzullo CL,
Sacchetti E, Galluzzo A, Parariello A, Colombo F, Zagliani A, Clerici M.
Cytokine profiles in drug-naïve schizophrenic patients. Schizophr Res
47:293-298, 2001.
161.
Vgontzas AN,
Papanicolaou DA, Bixler EO, Kales A, Tyson K, Chrousos GP. Elevation of plasma
cytokines in disorders of excessive daytime sleepiness: role of sleep
disturbance and obesity. J Clin Endocrinol Metab 82:1313-1316, 1997.
162.
Song C, Lin A,
Bonaccorso S, Heide C, Vererk R, Kenis G, Bosmans E, Scharpe S, Whelan A,
Cosyns P, de Jongh R, Maes M. The inflammatory response system and the
availability of plasma tryptophan in patients with primary sleep disorders and
major depression. J Affect Disord 49:211-219, 1998.
163.
Maes M, Song C,
Lin A, De Jongh RD, Gastel AV, Kenis G, Bosmans E, De Meester I, Benoy I, Neels
H, Demedts P, Janca A, Scharpe S, Smith RS. The effects of psychological stress
on humans: increased production of pro-inflammatory cytokines and a Th1-like
response in stress-induced anxiety. Cytokine 10:313-318, 1998.
164.
Maes M, Van
Bockstaele DR, Van Gastel A, Song C, Schotte C, Neels H, DeMeester I, Scharpe
S, Janca A. The effects of psychological stress on leukocyte subset
distribution in humans: evidence of immune activation. Neuropsychobiology
39:1-9, 1999.
165.
Maes M, Lin A-h,
Delmeire L, Van Gastel A, Kenis G, De Jongh R and Bosmans E. Elevated serum
interleukin-6 (IL-6) and IL-6 receptor concentrations in posttraumatic stress
disorder following assidental man-made traumatic events. Biol Psychiatry
45:833-839, 1999.
166.
Feldman M,
Brennan FM, Maini R. Cytokines in autoimmune diseases. Intern Rev Immunol
17:217-228, 1998.
167.
Odeh M. New
insights into the pathogenesis and treatment of rheumatoid arthritis. Clin
Immunol Immunopath 83:103-116, 1997.
168.
169.
Hotchkiss RS,
Karl IE. The pathophysiology and treatment of sepsis. N Engl J Med
348:2:138-150, 2003.
170.
van der Poll T and van Deventer SJH. Cytokines and
anticytokines in the pathogenesis of sepsis. Infectious Disease Clinics of
171.
Minneci P, Deans K, Natanson C, Eichacker PQ. Increasing the efficacy of antiinflammatory agents
used in the treatment of sepsis. Eur J Clin Microbiol Infect Dis 22:1-9, 2003.
172.
Siddiqi A, Monson
JP, Wood DF, Besser GM, Burrin JM. Serum cytokines and thyrotoxicosis. J Clin
Endocrinol Metab 84:435-439, 1999.
173.
Birks EJ,
174.
Plenz G, Eschert H, Erren M, Wichter T, Bohm M, Flesch M,
Scheld HH, Deng MC. The
interleukin-6/interleukin-6-receptor system is activated in donor hearts. J Am
Coll Cardiol 39:1508-1512, 2002.
175.
Holm T, Aukurst
P, aagaard E, Ueland T, Haugstad TS, Kjekshus J, Simonsen S, Froland SS,
Gullestad L, Asdreassen AK. Hypertension in relation to nitric oxide,
asymmetric dimethylarginine, and inflammation: different patters in heart
transplant recipients and individuals with essential hypertension.
Transplantation 74:10:1395-14, 2002.
176.
Rocha PN, Plumb
TJ, Coffman TM. Eicosanoids: lipid mediators of inflammation in
transplantation. Springer Semin Immunopathol 25:215-227, 2003.
177.
Bathgate A,
Pravica V, Therapondos G, Plevris JN, Hayes P,
178.
Kubala L, Ciz M,
Vondracek J, Cizova H, Cerny J, Nemec P, Studenik P, Duskova M, Lojek A. Peri- and
post-operative course of cytokines and the metabolic activity of neutophils in
human liver transplantation. Cytokine 16:3:97-101, 2001.
179.
Belperio JA,
DiGiovine B, Keane MP, Burdick MD, Ying Xue Y, Ross DJ, Lynch JP III, Kunkel
SL, Strieter RM. Interleukin-1 receptor antagonist as a biomarker for
bronchiolitis obliterans syndrome in lung transplant recipients.
Transplantation 73:4:591-599, 2002.
180.
Asderakis A,
Sankaran D, Dyer P, Johnson RWG, Praciva V, Sinnott PJ, Roberts I, Hutchinson
IV. Association of polymorphisms in the human interferon-g and interleukin-10 gene with acute and chronic kidney
transplant outcome. Transplantation 71:5:674-678, 2001.
181.
Juffermans NP,
Verbon A, van Deventer SJH, van Deutekom H, Speelman P, van der Poll T. Tumor
necrosis factor and interleukin-1 inhibitors as markers of disease activity in
tuberculosis. Am J Respir Crit Care Med 157:1328-1331,
1998.
182.
Evans CAW, Jellis
J, Hughes SPF, Remick DG, Friedland JS. Tumor necrosis factor-a, interleukin-6, and interleukin-8 secretion and the
acute-phase response in patients with bacterial and tuberculous osteomyelitis.
J Infect Dis 177:1582-1587, 1998.
183.
Nguyen TT, Gilpin DA, Meyer NA, Herndon DN. Current treatment of severly burned patients. Ann Surg
223:1:14-25, 1996.
184.
Gando S, Kameue
T, Matsuda N, Hayakawa M, Ishitani T, Morimoto Y and Kemmotsu O. Combined
activation of coagulation and inflammation has an important role in multiple
organ dysfunction and poor outcome after severe trauma. Thromb Haemost
88:943-949, 2002.
185.
Bonaccorso S,
Puzella A, Marino V, Pasquini M, Biondi M, Artini M, Almerighi C, Levrero M,
Egyed B, Bosmans E, Meltzer HY, Maes M. Immunotherapy with interferon-alpha in
patients affected by chronic hepatitis C induces an intercorrelated stimulation
of the cytokine network and an increase in depressive and anxiety symptoms.
Psychiatr Res 105:45-55.
186.
Capuron L, Ravaud
A, Dantzer R. Early depressive symptoms in cancer patients receiving
interleukin-2 and/or interferon alfa-2b therapy. J Clin Oncol 18:2143-2151,
2000.
187.
Capuron L, Ravaud
A, Gualde N, Bosmans E, Dantzer R, Maes M, Neveu PJ . Association between
immune activation and early depressive symptoms in cancer patients treated with
interleukin-2-based therapy. Psychoneuroendocrinology 26:797-808, 2001.
188.
Hurlimann D,
Forster A, Noll G, Enseleit F, Chenevard R, Distler O, Bechir M, Spieker LE,
Neidhart M, Michel BA, Gay RE, Luscher TF, Gay S, Ruschitzka F. Anti-tumor
necrosis factor-a treatment improves endothelial function in patients with rheumatoid
arthritis. Circulation 106:2184-2187, 2002.
189.
Wolfe F, Freundlich B, Straus WL. Increase in cardiovascular and cerebrovascular disease
prevalence in rheumatoid arthritis. J Rheumatol 30:36-40, 2003.
190.
Argiles JM,
Meijsing SH, Pallares-Trujillo J, Guirao X, Lopez-Soriano FJ. Cancer cachexia:
a therapeutic approach. Med Res Rev 21:1:83-101, 2001
191.
Mantovani G,
Maccio A, Massa E, Madeddu C. Managing cancer-related anorexia/cachexia. Drugs
61:4:499-514, 2001.
192.
Baracos VE.
Management of muscle wasting in cancer-associated cachexia. Cancer 92:1669-1677,
2001.
193.
Ionescu AA, Nixon
LS, Luzio S, Lewis-Jenkins V, Evans WD, Stone MD, Owens DR, Routledge PA, Shale
DJ. Pulmonary function, body composition, and protein catabolism in adults with
cystic fibrosis. Am J Respir Crit Care Med
165:495-500, 2002.
194.
Tisdale MJ.
Cachexia in cancer patients. Nat Rev Cancer 2:862-871, 2002.
195.
van Crevel R, Karyadi E, Netea MG, Verhoeff H, Nelwan
RHH, West CE and van der Meer JWM. Decreased plasma leptin concentrations in
tuberculosis patients are associated with wasting and inflammation. J Clin
Endocrinol Metab 87:758-763, 2002.
196.
Argiles JM, Lopez-Soriano FJ. The role of cytokines in cancer cachexia. Med Res Rev
19:223-248, 1999.
197.
Moldawer LL,
Copeland EM. Proinflammatory cytokines, nutritional support, and the cachexia
syndrome. Cancer 79:1828-1839, 1997.
198.
Dentino AN,
Pieper CF, Rao MK, Currie MS, Harris T, Blazer DG, Cohen HJ. Association of
interleukin-6 and other biologic variables with depression in older people
living in the community. JAGS 47:6-11, 1999.
199.
Jenny NS, Tracy
RP, Ogg MS, Luong LA, Kuller LH,
200.
Biasucci LM,
Liuzzo G, Fantuzzi G, Caligiuri G, Rebuzzi AG, Ginnetti F,
201.
Verheggen PWHM,
de Maat MPM, Manger Cats V, Haverkate F, Zwinderman AH, Kluft C, Bruschke AVG.
Inflammatory status a a main determinant of outcome in patients with unstable
angina, independent of coagulation activation and endothelial cell function.
Eur Heart J 20: 567-574, 1999.
202.
Buffon A, Liuzzo
G, Biasucci LM, Pasqualetti P, Ramazzotti V, Rebuzzi AG, Crea F, Maseri A.
Preprocedural serum levels of C-reactive protein predict
early complications and late restenosis after coronary angioplasty. J Am Coll
Cardiol 34:1512-1521, 1999.
203.
Kosuge M, Kimura
K, Ishikawa T, Endo T, Shigemasa T, Okuda J, Sugiyama M, Tochikubo O, Umemura
S. Relation between C-reactive protein levels on admission and pattern of acute
myocardial infarction onset. Am J Cardiol 86:83-86, 2000.
204.
Rossi E, Biasucci
LM, Citterio F, Pelliccioni S, Monaco C, Ginnetti F, Angiolollo DJ, Grieco G,
Liuzzo G, Maseri A. Risk of myocardial infarction and angina in patients with
severe peripheral vascular disease predictive role of C-reactive protein.
Circulation 105:800-803, 2002.
205.
Zairis MN,
Manousakis SJ, Stefanidis AS, Papdaki OA, Andrikopoulos GK, Olympios CD,
Hadjissavas JJ, Argyrakis SK, Foussas SG. C-reactive protein levels on
admission are associated with response to thrombolysis and prognosis after ST-segment
elevation acute myocardial infarction. Am Heart J 144:782-789, 2002.
206.
Ridker PM, Cannon
CP, Morrow D, Rifai N, Rose LM, McCabe CH, Pfeffer MA, Braunwald E for the
pravastatin or atorvastatin evaluation and infection therapy-thrombolysis in
myocardial infarction 22 (PROVE IT-TIMI 22) Investigators. C-reactive protein
levels and outcomes after statin therapy. N Engl J Med. 352:20-28, 2005.
207.
Nissen SE, Tuzcu
EM, Schoenhagen P, Crowe T, Sasiela WJ, Tsai J, Orazem J, Magorien RD,
O’Shaughnessy C, Ganz P for the reversal of atherosclerosis with aggressive
lipid lowering (REVERSAL) investigators. Statin therapy, LDL cholesterol,
C-reactive protein, and coronary artery disease. N Engl J Med. 352:29-38, 2005.
208.
Saito M,
Ishimitsu T, Minami J, Ono H, Ohrui M, Matsuoka H. Realtions of plasma
high-sensitivity C-reactive protein to traditional cardiovascular risk factors.
Atherosclerosis. 167:73-79, 2003.
209.
van Wissen S, Trip MD, Smilde TJ, de Graaf J, Stalenhoef
AFH, Kastelein JJP. Differential hs-CRP
reduction in patients with familial hypercholesterolemia treated with
aggressive or conventional statin therapy. Atherosclerosis. 165:361-366, 2002.
210.
de Maat MPM, Trion A. C-reactive protein as a risk
factor versus risk marker. Curr Opin Lipidol 15:651-657.
211.
Kosuge M, Kimura
K, Ishikawa T, Endo T, Shigemasa T, Okuda J, Sugiyama M, Tochikubo O, Umemura
S. Relation between C-reactive protein levels on admission and pattern of acute
myocardial infarction onset. Am J Cardiol 86:83-86, 2000.
212.
Lagrand WK,
Visser CA, Hermens WT, Niessen HWM, Verheugt FWA, Wolbink GJ, Hack E.
C-reactive protein as a cardiovascular risk factor more than an epiphenomenon?
Circulation 100:96-102, 1999.
213.
Pietila KO,
Harmoinen AP, Jokinitty J, Pasternack AI. Serum C-reactive protein
concentration in acute myocardial infarction and its relationship to mortality
during 24 months of follow-up in patients under thrombolytic treatment. Eur
Heart J 17:1345-1349, 1996.
214.
Biasucci L,
Liuzzo G, Grillo RL, Caligiuri G, Rebuzzi AG, Buffon A, Summaria F, Ginnetti F,
Fadda G, Maseri A. Elevated levels of C-reactive protein at discharge in
patients with unstable angina predict recurrent instability. Circulation 99:855-860,
1999.
215.
Rohde LEP, Hennekens CH, Ridker PM. Survey of C-reactive protein and cardiovascular risk
factors in apparently healthy men. Am J Cardiol 84:1018-1022, 1999.
216.
Strandberg TE,
Tilvis RS. C-reactive protein, cardiovascular risk factors, and mortality in a
prospective study in the elderly. Arterioscler Thromb Vasc Biol 20:1057-1060,
2000.
217.
Tice JA, Browner
W, Tracy RP, Cummings SR. The relation of C-reactive protein levels to total
and cardiovascular mortality in older U.S. Women. Am J
Med 114:199-205, 2003.
218.
Warzocha K,
Salles G, Bienvenu J, Bastion Y, Dumontet C, Renard N, Neidhardt-Berard E-M,
Coiffier B. Tumor nercosis factor ligand-receptor system can predict treatment
outcome in lymphoma patients. J Clin Oncol 15:499-508, 1997.
219.
220.
Aukhurst P,
Muller F, Lien E, Nordoy I,
221.
Lew E, Gallagher
L, Kuehnert M, Rimland D, Hubbard M, Parekh B, Zell E, Jarvis W, Jason J.
Intracellular cytokines in the acute response to highly active retroviral
therapy. AIDS 15:1665-1670, 2001.
222.
Matsuse H,
Shimoda T, Matuso N, Obase Y, Asai S, Kohno S. Corticosteroid resistance in
mild asthma: markers of persistent inflammation. Ann Allergy Asthma Immunol
82:457-462, 1999.
223.
Kondo S, Yazawa
H, Jimbow K. Reduction in serum interleukin-5 levels reflect
clinical improvement in patients with atopic dermatitis. J Dermatol 28:237-243,
2001.
224.
Fujii K, Konishi
K, Kanno Y, Ohgou. Acute urticaria with elevated circulating interleukin-6 is
resistant to anti-histamine treatment. J Dermatol 28:248-250, 2001.
225.
Kobayashi D,
Watanabe N, Yamauchi N, Tsuji N, Sato T, Nitsu Y. Endogenous tumor necrosis
factor as a predictor of doxorubicin sensitivity in leukemic patients. Blood
89:7:2472-2479, 1997.
226.
Teicher BA,
Kakeji Y, Ara G, Herbst RS, Northey D. Prostrate carcinoma response to
cytotoxic therapy: in vivo
resistance. in vivo 11:453-462, 1997.
227.
Arteaga CL, Koli
KM, Dugger TC, Clarke R. Reversal of tamoxifen resistance of human breast
carcinomas in vivo by neutralizing
antibodies to transforming growth factor-b. J Natl Cancer Inst 91:46-53, 1999.
228.
Makino T, Noguchi
Y, Yoshikawa T, Doi C, Nomura K. Circulating interleukin 6 concentrations and
insulin resistance in patients with cancer. Br J Sur 85:1658-1662, 1998.
229.
Inagawa H,
Nishizawa T, Honda T, Nakamoto T, Takagi K, Soma GI. Mechanisms by which
chemotherpeutic agents augment the antitumor effects of tumor necrosis factor:
involvement of the pattern shift of cytokines from Th2 to Th1 in tumor lesions.
Anticancer Research 18:3957-3964,
1998.
230.
Mayo P, Skeith K,
Russell AS, Jamali F. Increased S-verapamil plasma concentration in rheumatic
arthritis without increased cardiac effect. Br J Clin Pharmacol 50:605-613,
2000.
231.
Ridker PM, Rifai
N, Pfeffer MA, Sacks F, Braunwald E. Long-term effects of pravastatin on plasma
concentration of C-reactive protein. Circulation 100:230-235, 1999.
232.
Kwak B, Mulhaupt
F, Myit S, Mach F. Statins as a newly recognized type of immunomodulator.
Nature Med 6:12:1399-1402, 2000.
233.
Saghizadeh M, Ong
JM, Garvey WT, Henry RR, Kern PA. The expression of TNFa by human muscle. J Clin Invest 97:4:1111-1116, 1996.
234.
Abernethy DR, Schwartz
JB, Todd EL, Luchi R, Snow E. Verapamil pharmacodynamics and disposition in
young and elderly hypertensive patients altered electrocardiographic and
hypotensive responses. Ann Intern Med 105:329-336, 1986.
235.
Abernethy DR,
Wainer IW, Longstreth JA,
236.
MRC Working
Party. Medical Research Council trial of treatment of hypertension in older
adults: principal results. BMJ 304:405-412, 1992.
237.
Messerli FH,
Grossman E,
238.
Manandhar R, Shrestha N, Butlin CR, Roche PW. High levels of inflammatory cytokines are associated
with poor clinical response to steroid treatment and recurrent episodes of type
1 reactions in leprosy. Clin Exp Immunol 128:333-338, 2002.
239.
Silverman HJ, Penaranda R, Orens JB, Lee NH. Impaired beta-adrenergic receptor stimulation of
cyclic adenosine monophosphate in human septic shock: association with
myocardial hyperresponsiveness to catecholamines. Crit Care Med 21:31-39, 1993.
240.
Bekker LG,
Maartens G, Steyn L, Kaplan G. Selective increase in plasma tumor necrosis
factor-a and concomitant clinical
deterioration after initiating therapy in patients with severe tuberculosis. J
Infect Dis 178:580-584, 1998.
241.
Abernethy DR, Schwartz JB. Verapamil pharmacodynamics and
disposition in obese hypertensive patients. J
Cardiovasc Pharmacol 11:209-215, 1988.
242.
Szabo I, Chen XH,
Xin L, Adler MW, Howard OMZ, Oppenheim JJ,
243.
Maes M, Bosmans
E, De Jong R, Kenis G, Vandoolaeghe E, Neels H. Increased serum IL-6 and IL-1
receptor antagonist concentrations in major depression and treatment resistant
depression. Cytokine 9:853-858, 1997.
244.
Lin A, Kenis G,
Bignotti S, Tura GJB, De Jong R, Bosmans E, Pioli R, Altamura C, Scharpe S,
Maes M. The inflammatory response system in treatment-resistant schizophrenia:
increased serum interleukin-6. Schizophr Res 32:9-15, 1998.
245.
Cazzullo CL,
Sacchetti E, Galluzzo A, Panariello A, Adorni A, Pegoraro M, Bosis S, Colombo
F, Trabattoni D, Zagliani A, Clerici M. Cytokine profiles in schizophrenic
patients treated with risperidone a 3-month follow-up study. Prog
Neuropsychopharmacol Biol Psychiatry 26:33-39, 2002.
246.
Maes M, Chiavetto
LB, Bignotti S, Tura GJB, Piolo R, Boin F, Kenis G, Bosmans E, de Jongh R,
Altamura CA. Increased serum interleukin-8 and interleukin-10 in schizophrenic
patients resistant to treatment with neuroleptics and the stimulatory effects
of clozapine on serum leukemia inhibitory factor receptor. Schizophr Res
54:281-291, 2002.
247.
Kim WU, Cho ML,
Kim SI, Yoo WH, Lee SS, Joo YS, Min JK, Hong YS, Lee SH, Park SH, Cho CS, Kim
HY. Divergent effect of cyclosporine on Th1/Th2 type cytokines in patients with
severe, refractory rheumatoid arthritis. J Rheumatol 27:324-321, 2000.
248.
Yudoh K, Matsuno
H, Nakazawa F, Yonezawa T, Kimura T. Increased expression of multidrug
resistance of P-glycoprotein on Th1 cells correlates with drug resistance in
rheumatoid arthritis. Arthritis Rheum 42:2014-2015, 1999.
249.
Schneider RE, Bishop H, Kendall MJ, Quaterman CP. Effect of inflammatory disease on plasma
concentrations of three b-adrenoceptor blocking agents. Int J of Clin Pharmacol Ther Toxicol
19:158-162, 1981.
250.
Emami J, Pasutto
FM, Jamali F. Effect of experimental diabetes mellitus and arthritis on the
pharmacokinetics of hydroxychloroquine enantiomers. Pharm Res 15:6:897-903,
1998.
251.
Piquette-Miller
M, Jamali F. Influence of severity of inflammation on the disposition kinetics
of propranolol enantiomers in ketoprofen-treated and untreated adjuvant rats.
Drug Metab Dispos 23:240-245, 1995.
252.
Belpaire FM, De
Smet F, Chindavijak B, Fraeyman N, Bogaert MG. Effect of turpentine-induced
inflammation on the disposition kinetics of propranolol, metoprolol, and
antipyrine in the rat. Fundam Clin Pharmacol 3:79-88, 1989.
253.
Piafsky KM, Borga
O, Odar-Cederlof I, Johansson C, Sjoqvist F. Increased plasma protein binding
of propranolol and chlorpromazine mediated by disease-induced elevations of
plasma a1-acid glycoprotein. N Eng J Med 299:1435-1439, 1978.
254.
Morgan ET.
Regulation of cytochromes P450 during inflammation and infection. Drug Metab
Rev 29:1129-1188, 1997.
255.
Muller CM,
Scierka A, Stiller RL, Kim Y-M, Cook DR, Lancaster JR, Buffington CW, Watkins
WD. Nitric oxide mediates hepatic cytochrome P450 dysfunction induced by
endotoxin. Anesthesiology 84:1435-1442, 1996.
256.
Piquette-Miller
M, Jamali F. Selective effect of adjuvant arthritis on the disposition of
propranolol enantiomers in rats detected using a stereospecific HPLC assay.
Pharm Res 10:294-299, 1993.
257.
Guirguis MS,
Jamali F. Disease-Drug interaction: reduced response to propranolol despite
increased concentration in the rat with inflammation. J Pharm Sci 92:1077-1084,
2003.
258.
Lui SJ, Zhou W,
Kennedy RH. Suppression of β-adrenergic responsiveness of L-type Ca2+
current by IL-1β in rat ventricular myocytes. Am J Physiol 276:H141-148, 1999.
259.
Gulick T, Chung MK, Pieper SJ, Lange LG, Schreiner GF. Interleukin-1 and tumor necrosis factor inhibit
cardiac myocyte β-adrenergic responsiveness. Proc Natl Acad Sci
260.
Davies CH,
Ferrara N, Harding SE. β-adrenoceptor function changes with age of
subjects in myocytes from non-failing human ventricle. Cardiovasc Res
31:152-156, 1996.
261.
Krown KA, Yasui
K, Brooker MJ, Dubin AE, Nguyen C, Harris GL, McDonough PM, Glembotski CC,
Palade PT, Sabbadini RA. TNFa receptor expression in rat cardiac myocytes: TNFa inhibition of L-type Ca2+ current and Ca2+
transients. FEBS Lett 376:24-30, 1995.
262.
Liu S and Schreur
KD. G protein-mediated suppression of L-type Ca2+ current by
interleukin-1β in cultured rat ventricular myocytes. Am J Physiol
268:C339-349, 1995.
263.
Nishio M, Habuchi
Y, Tanaka H, Morikawa J, Okanoue T, Kashima K. Tyrosine kinase-dependent
modulation by interferon-a of the ATP-sensitive K+ current in rabbit ventricular
myocytes. FEBS Lett 445:87-91, 1999.
264.
Kulmatycki KM,
Abouchehade K, Sattari S, Jamali F. Drug-disease interactions: reduced b-adrenergic and potassium channel antagonist
activities of sotalol in the presence of acute and chronic inflammatory conditions.
Br J Pharmacol 133:286-294, 2001.
265.
Abouchehade KD,
Russell AS, Jamali F. Anti-TNF treatment normalizes inflammation and
upregulates the responses to atenolol. AAPSPharm Sci 3:3, 2001.
266.
Guirguis MS,
Jamali F. The effect of anti-TNF therapy on propranolol pharmacodynamics in
adjuvant arthritic rats. AAPSPharm Sci 3:3, 2001.
267.
Jacobsson LT,
Turesson C, Gulfe A, Kapetanovic MC, Petersson IF, Saxne T, Geborek P.
Treatment with tumor necrosis factor blockers is associated with a lower
incidence of first cardiovascular events in patients with rheumatoid arthritis.
J Rheumatol 32:1213-1218, 2005.
268.
Chung ES, Packer M, Lo KH, Fasanmade AA, Willerson JT. Results of the anti-TNF therapy against congestive
heart failure (ATTACH) trial. Randomized, double-blind, placebo-controlled,
pilot trial of infliximab, a chimeric monoclonal antibody to tumor necrosis
factor-a, in patients with
moderater-to-severe heart failure. Circulation 107:3133-3140, 2003.
269.
Sattari S, Dryden
WF, Eliot LA, Jamali F. Despite increased plasma concentration, inflammation
reduced potency of calcium channel antagonists due to lower binding to the rat
heart. Br J Pharmacol 139:945-954, 2003.
270.
Daneshtalab N,
Lewanczuk RZ, Russell A, Jamali F. Rheumatoid arthritis does not reduce the
pharmacodynamic response to valsartan. J Clin Pharmacol 44:245-252, 2004.
271.
Gurantz D,
Cowling RT, Villarreal FJ, Greenberg BH. Tumor necrosis factor-alpha
upregulates angiotensin II type 1 receptors on cardiac
fibroblasts. Circ res 85:272-279, 1999.
272.
Kaprielian RR,
Dupont E, Hafizi S, Poole-Wilson PA, Khaghani A, Yacob MH, Severs NJ.
Angiotensin II receptor type 1 mRNA is upregulated in atria of patients with
end-stage heart failure. J Mol Cell Cardiol 29:2299-2304, 1997.
273.
Peng JF, Gurantz
D, Tran V, Cowling RT, Greenberg BH. Tumor necrosis factor-a-induced AT1 receptor upregulation enhances
angiotensin II-mediated cardiac fibroblast responses that
favors fibrosis. Cir Res 91:1119-1126, 2002.
274.
de Boer RA, Pinto
YM, Suurmeijer AJ, Pokharel S, Scholtens E, Humler M, Saavedra JM, Boomsma F,
van Gilst WH, van Veldhuisen DJ. Increased expression of cardiac angiotensin II
type 1 (AT1) receptors decreases myocardial microvessel density
after experimental myocardial infarction. Cardiovasc Res 57:434-442, 2003.
275.
Slaviero KA,
Clarke SJ, Rivory LP. Inflammatory response: an unrecognized source of
variability in the pharmacokinetics and pharmacodynamics of cancer
chemotherapy. Lancet Oncol 4:224-232, 2003.
276. Ariyo AA, Haan M, Tangen CM, Rutledge JC, Cushman M, Dobs A, Furberg CD. Depressive Symptoms and Risks of Coronary Heart Disease and Mortality in Elderly Americans. for the Cardiovascular Health Study Collaborative Research Group. Circulation 102:1773-1779, 2000.
Published by the Canadian Society for Pharmaceutical Sciences.
Copyright © 1998 by the Canadian Society for Pharmaceutical Sciences.
CSPS Home | JPPS Home | Search | Subscribe to JPPS