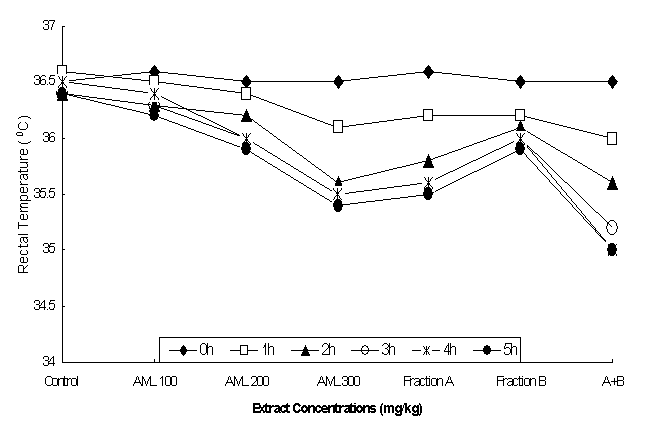
Figure
1. Effect of different
concentrations of A.macrophylla
extract on normal body temperature of Rats.
J Pharm Pharmaceut Sci (www.cspscanada.org) 8(3):558-564, 2005
Antipyretic Activity of Alstonia macrophylla Wall ex
Debprasad Chattopadhyay1, Ganeshan Arunachalam1, Lopamudra Ghosh2, Rajendran K.3, Asit B. Mandal4, S. K. Bhattacharya1, 3
1ICMR Virus Unit, Infectious
2Division of Pharmacognosy,
Department of Pharmaceutical Technology,
3National
4Biotechnology Laboratory, Central Agricultural Research Institute,
Received January 27, 2005; Revised September 29, 2005; Accepted September 30, 2005; Published October 6, 2005.
Corresponding Author:
D. Chattopadhyay, Senior Research Officer, ICMR Virus Unit, Infectious Diseases & Beliaghata General
Hospital, GB 4, 1st Floor, 57, Beliaghata Main Road, Kolkata 700
010.
debprasadc@yahoo.co.in;
icmrvubd@vsnl.net
ABSTRACT.
Purpose. Alstonia
macrophylla Wall ex
Alstonia macrophylla Wall ex
The leaves
of Alstonia macrophylla Wall ex A. DC
were collected from the rain forests of Middle and South Andamans, India,
during April, June and October 1999 and February, August and November 2000. The
voucher specimens have been identified (Herbarium No. 9220, 9221, 9227 and 9228
respectively) and deposited at the Herbarium Section of the Botanical Survey of
India, Andaman & Nicobar Circle, Port Blair, India.
Extraction
and fractionation
Coarsely powdered dry leaves (1 kg) were
successively extracted with 95% MeOH at room temperature for 72 h. The whole
extract was filtered and the solvent were evaporated to dryness in vacuo to a
residue with an Eyela Rotary Evaporator (
Adults Wistar albino rats of either
sex weighing 180-200 g each were used. The animals were kept in the standard
metal cages in groups of 10 per cage, with free access to standard diet and
water ad libitum in the animal house
of the Pharmaceutical Technology Department,
Rats of either sex were divided into
seven groups, comprising six in each group. The body temperature of each rat
was measured rectally at predetermined time intervals before and for 5h after
the administration of either propylene glycol (vehicle control) or AML extract
at doses of 100, 200 and 300 mg/kg, fractions A and B at 50 mg/kg and a
combination of fractions A and B (50 + 50 mg/kg body weight) orally.
Yeast
induced pyrexia was used to evaluate the antipyretic activity of the extract.
The rats were divided into eight groups of six animals each and the body
temperature of each rat was recorded by measuring rectal temperature at
predetermined time intervals. Fever was induced by injecting 15% suspension of
Brewer’s yeast (Saccharomyces cerevisiae),
following a standard method (9). In brief, the rats were allowed to remain
quiet in the cage for sometimes. A thermister probe was inserted 3-4 cm deep
into the rectum, after fastened the tail, to record the basal rectal
temperature. The animals were then given a subcutaneous injection of 10 ml/kg
of 15% w/v Brewer’s yeast suspended in 0.5% w/v methylcellulose solution and
the animals were returned to their housing cages. 19h after yeast injection,
the rats were again restrained in individual cages to record their rectal
temperature. Immediately the AML extract was administered orally at doses of
100, 200 and 300 mg/kg to the first three groups of animals, the
fourth and fifth group received 50 mg/kg of fraction A and B respectively,
while the sixth group received a combination of both the fractions A (50 mg/kg)
and B (50 mg/kg), the seventh group received 5 ml/kg of propylene
glycol as vehicle control and the last group was administered with 150 mg/kg of
paracetamol (for each rat 27 mg of paracetamol was dissolved in 0.5 ml
propylene glycol and diluted with 4.5 ml distilled water) as drug control. Rectal temperature of all the rats was
recorded at 19 h, immediately before extract or vehicle or paracetamol
administration, and again at 1h interval upto 23h, after yeast injection (10).
The statistical analysis was carried out with SPSS 13.0 (Windows) software. Difference of the parametric data of body temperature(s) was examined by two-way analysis of variance (ANOVA) with Dunett’s Post hoc pair wise multiple comparison t test, to compare a set of experimental data against control mean.
The effect of AML extract on normal
body temperature in rats is presented in
Figure 1. The results showed that the
leaf extract at doses of 200 mg/kg caused significant lowering of the body
temperature up to 3 h following extract administration, as the normal mean
temperature 36.50C at 0 h was reduced to 36.00C at 3 h.
While maximum lowering of body temperature was noticed at 300 mg/kg of the leaf
extract, as the mean temperature of 36.50C was reduced to 35.40
C within 3-5h period in a dose-dependent manner.
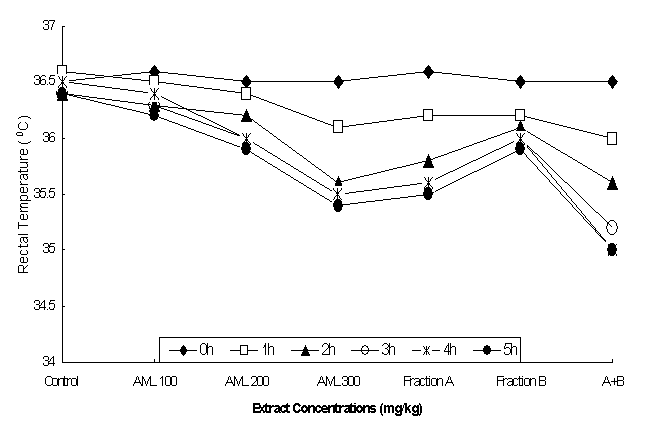
Figure
1. Effect of different
concentrations of A.macrophylla
extract on normal body temperature of Rats.
Table 1. Statistical Analysis by SPSS 13.0 on Normal
Body temperature
|
Body |
2-WAY ANOVA Dunnett t (2-sided) Post Hoc test, compare with
vehicle control group (½MD½±SE) |
|||||
|
Methanol extract of Alstonia
macrophylla leaf |
Fractions |
|||||
|
100 mg/kg |
200 mg/kg |
300 mg/kg |
A (50 mg/ kg) |
B (50 mg/ kg) |
A+B |
|
|
0hrs |
0.100 ±
.0414 |
0.000 ±
.0414 |
0.000 ±
.0414 |
0.100 ±
.0414 |
0.000 ±
.0414 |
0.000 ±
.0414 |
|
1hrs |
0.100 ±
.0390 |
0.200 ±
.0390* |
0.300 ±
.0390* |
0.400 ±
.0390* |
0.400 ±
.0390* |
0.600 ±
.0390* |
|
2hrs |
0.100 ±
.0451 |
0.200 ±
.0451* |
0.833 ±
.0451* |
0.600 ±
.0451* |
0.300 ±
.0451* |
0.800 ±
.0451* |
|
3hrs |
0.100 ±
.0414 |
0.400 ±
.0414* |
0.900 ±
.0414* |
0.800 ±
.0414* |
0.400 ±
.0414* |
1.200 ±
.0414* |
|
4hrs |
0.100 ±
.0436 |
0.500 ±
.0436* |
1.000 ±
.0436* |
0.900 ±
.0436* |
0.500 ±
.0436* |
1.500 ±
.0436* |
|
5hrs |
0.200 ±
.0390* |
0.500 ±
.0390* |
1.000 ±
.0390* |
0.900 ±
.0390* |
0.500 ±
.0390* |
1.400 ±
.0390* |
*p=<0.001, MD=Mean
difference, SE= Standard Error.
The interesting finding is the effect
of combination of fraction A and B at 50 mg/kg each, showing maximum lowering
of temperature as the initial temperature 36.50C is reduced to 35.00C
within 4-5h period, as shown in Figure 1. The results of 2-way ANOVA showed
that there was significant differences among the extract treated groups
(F=765.15, p<0.001), body temperatures (F=722.30, p<0.001 and interaction
of different concentration of the extract and fractions (F=48.33, p<0.001)
in maintaining normal body temperature (Table 1).
The effect of AML extract on yeast-induced pyrexia is presented in Figure 2. The data revealed that the rectal temperature of 37.30C at 0h was markedly elevated to 39.60C for vehicle control and 39.00C for paracetamol control group 19h after the subcutaneous injection of yeast suspension. The animals treated with AML extract at 100, 200 and 300 mg/kg doses showed a decrease in the rectal temperature by 0.5, 0.6 and 1.30C respectively within 1h. On the otherhand, in 21h the temperature was reduced by 0.60C for all the groups of animals received AML extracts; and at 22h the temperature was reduced by 0.8 to 1.00C. While the recorded temperature 38.50C, 38.00C and 37.50C for 100, 200 and 300 mg/kg group in 23rd h showed that the extract can significantly reduced the temperature by 0.9, 1.2, 1.50C respectively.
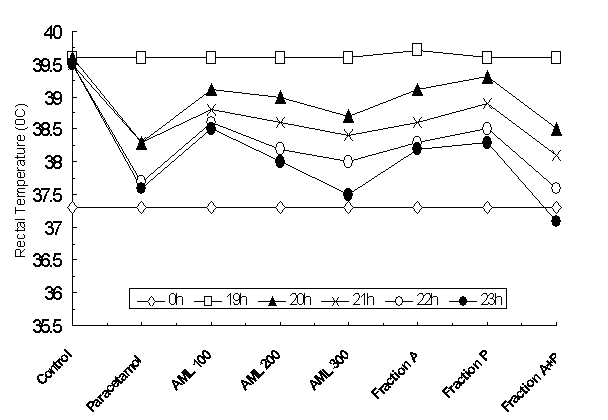
Figure 2. Effect of different concentrations of A.macrophylla leaf extract on
yeast-induced pyrexia in rats.
Table 2. Statistical Analysis by SPSS 13.0 on
Yeast-induced pyrexia
|
Rectal
Temp |
2-WAY
ANOVA Dennett t (2-sided) Post Hoc test, compare with vehicle control group (½MD½±SE) |
||||||
|
Methanol
extract of Alstonia macrophylla
leaf |
Fractions |
Paracetamol
(150 mg/kg) |
|||||
|
100
mg/kg |
200
mg/kg |
300
mg/kg |
A
(50 mg/kg) |
B
(50 mg/kg) |
A+B |
||
|
0hrs |
0.0 ± .0408 |
0.0 ± .0408 |
0.0 ± .0408 |
0.0 ± .0408 |
0.0 ± .0408 |
0.0 ± .0408 |
0.0 ± .0408 |
|
19hrs |
0.0 ± .0447 |
0.0 ± .0447 |
0.0 ± .0447 |
0.0 ± .0447 |
0.0 ± .0447 |
0.0 ± .0447 |
0.0 ± .0447 |
|
20hrs |
0.500 ± .0396* |
0.600 ± .0396* |
0.900 ± .0396* |
0.500 ± .0396* |
0.317 ± .0396* |
1.100 ± .0396* |
1.300 ± .0396* |
|
21hrs |
0.700 ± .0365* |
0.900 ± .0365* |
1.100 ± .0365* |
0.900 ± .0365* |
0.600 ± .0365* |
1.400 ± .0365* |
1.200 ± .0365* |
|
22hrs |
0.900 ± .0465* |
1.300 ± .0465* |
1.500 ± .0465* |
1.200 ± .0465* |
1.000 ± .0465* |
1.900 ± .0465* |
1.800 ± .0465* |
|
23hrs |
1.000 ± .0387* |
1.500 ± .0387* |
2.000 ± .0387* |
1.300 ± .0387* |
1.200 ± .0387* |
2.400 ± .0387* |
1.900 ± .0387* |
*p=<0.001, MD=Mean difference, SE= Standard Error.
However, the paracetamol (150 mg/kg) treated group showed that the rectal temperature of 39.00C was reduced by 0.70C at 21h and 1.40C at 23h respectively. The antipyretic effect of leaf extract at 300 mg/kg is similar to the paracetamol group. Interestingly the fraction A and B in combination, showed maximum reduction in temperature, from 39.00C to 37.10C within 5h of extract administration. This signifies that rectal temperature of the treated rats was decreased in a dose-dependent manner by AML extract. Furthermore, the antipyretic effect was started within 1h of extracts administration, and maintained for at least 5 h after the administration of the extract. The statistical analysis also revealed that the temperature difference was significant among the extract treated groups (F=909.02, p<0.001), body temperatures (F=5831.97, p<0.001) and different concentration of the extract and fractions interaction (F=115.83, p<0.001) to reduce the elevated temperature.
To
determine the role of extract and or fractions in restoring normal body
temperature or reducing the elevated body temperature of the treated animals,
the Dunett’s Post hoc pair wise when compared with the control group, the
analysis indicated that the mean differences were statistically significant on treatment
groups of AML 200 and 300 mg/kg (Table 1 and
Table 2). Moreover, the highest
reduction in body temperature was found with 300 mg/kg dose of AML extract; and
the administration of both the fraction (A and B) in combination yielded
highest significance level in comparison with paracetamol group.
Search for herbal remedies with potent
antipyretic activity received momentum recently as the available antipyretics,
such as paracetamol, nimusulide etc. have toxic effect to the various organs of
the body (11). The body’s ability to maintain a
natural balance of COX 1 and 2 that regulate inflammatory response play a
crucial role in supporting cardiovascular, immune, neurological, and joint and
connective tissue systems (7). A number of plant extracts modulate enzymes of
cyclooxygenase pathway, as reported with the rosmarinic acid of Rosmarinus officinalis that inhibit
leukotriene and prostaglandins synthesis, while COX-1 and COX-2 was inhibited by
cirsilineol, cirsimaritin, apigenin, rosmarinic acid and eugenol of Ocimum sanctum similar to ibuprofen, naproxen, and aspirin (12).
The
results showed that the methanol extract of AML possesses a significant
antipyretic effect in maintaining normal body temperature and reducing yeast-induced
elevated body temperature in rats in a dose dependent manner and its effect is
comparable to that of the standard antipyretic drug paracetamol. Furthermore,
the significant reduction of yeast provoked elevated temperature of the tested
animals by the extract at 200 mg/kg dose or more and 50 mg/kg of fractions
appears to be due to the action of ursolic acid, β-sitosterol and its
glucoside alone or in combination, as the maximum antipyretic effect was found
in combination of fraction A (β-sitosterol) and fraction B (ursolic acid).
Moreover, the statistical analysis with two-way ANOVA showed that the methanol
extract of AML decreases both the normal and yeast elevated body temperature in
a short span of time in a dose dependent manner, when compared with control
group. The interesting finding in yeast-induced experiment is that the fraction
A and B in combination was more effective than the paracetamol. The bioactive fraction
A contains b-sitosterol, a phytosterol with an extra
alkyl group at C-24 in the side chain. The b-sitosterol is a plasminogen
activator and promotes the formation of essential polyunsaturated fatty acids
from linoleic acid, required for prostaglandin and leukotriene synthesis (13). Beta-sitosterol
and its glycoside possess potent anti-inflammatory and antipyretic activity
(14), by reducing the secretion of pro-inflammatory cytokines and TNF-α
(14, 15). In vitro, animal, and human studies have shown that β-sitosterol
and its glucosides in combination selectively enhance the activity of helper-T
cells with a significant rise in interleukin 2 (IL-2) and gamma interferon
(IFN-γ) level, with enhanced natural killer (NK) cell activity and thus,
are therapeutically useful in immune dysfunction diseases (15). These phytosterols can enhance “adaptive” immunity through the
stimulation of “innate” immune system, and hence termed as the ‘adaptogen’
which promote overall health without the side effect and rapid response of
drugs (16). On the otherhand, fraction B of AML extract contains ursolic acid
as major constituent (3). The ursolic acid, a pentacyclic triterpene, has
diverse pharmacological actions including antiinflammatory (4, 17-19),
antihistaminic (20) and analgesic (17, 21). As a potent antiinflammatory agent
it inhibit human leukocyte elastage (22), 5-lipooxygenase and cyclooxygenase (12,
23) and thereby prostaglandin biosynthesis (24). Ursolic acid is also a potent
and highly selective inhibitor of cyclic AMP-dependent protein kinase and
phosphodiestarase and thereby regulates metabolism, cell division and gene
expression (25, 26).
It was evident from the study that the observed antipyretic effects of the extract were similar in both magnitude and time course. However, to know the exact mechanism of action of Alstonia macrophylla leaf extract further study with purified fractions is warranted.
The Authors wish to acknowledge the financial assistance of the Department of Biotechnology, Government of India, through the Grant No. BT/PRO237/OSC17 /005/ 96. Thanks are due to the Officer In-Charge, Botanical Survey of India, Andaman & Nicobar Circle, Port Blair, for identification of the plant. We express our sincerest gratitude to the Officer In-Charge, ICMR Virus Unit, Kolkata for his constant encouragement, help and suggestions.
1.
Bhargava N. Ethnobotanical studies of the
tribes of Andaman and Nicobar Islands, India. I. Onge. Econ Bot, 37: 110-119, 1983.
2.
Asolkar, LV.; Kakkar, KK.; Chakre, OJ., (ed.). Second
supplement to Glossary of Indian
Medicinal Plants with active principles, Part 1, Publications & Information
Directorate, CSIR, New Delhi, India, pp. 51-52, 1992.
3.
Chattopadhyay
D, Maiti K, Kundu AP, Chakrabarty MS, Bhadra R, Mandal SC, Mandal AB.
Antimicrobial activity of Alstonia
macrophylla: A folklore of Bay Islands. J
Ethnopharmacol, 71: 49-55, 2001.
4.
Arunachalam
G, Chattopadhyay D, Chatterjee S, Mandal AB, Sur TK, Mandal SC. Evaluation of
antiinflammatory activity of Alstonia
macrophylla Wall ex A. DC. Leaf extract. Phytomedicine, 9(7): 632-635, 2002.
5.
Spacer
CB, Breder CD. The neurologic basis of fever. New England J Med, 330: 1880-1886, 1994.
6.
Veugelers PJ, Kaldor JM, Strathdee SA, Page-Shafer
KA, Schechter MT, Coutinho RA, Keet IP, van Griensven GJ. Incidence and prognostic significance of symptomatic
primary human immunodeficiency virus type 1 infection in homosexual men. J Infect Dis, 176: 112-117, 1997.
7.
Cheng L,
Ming-liang H, Lars B. Is COX-2 a perpetrator or a protector? Selective COX-2
inhibitors remain controversial. Acta
Pharmacologica Sinica, 26 (8): 926-933, 2005.
8.
Trease,
GE.; Evans, WC., Pharmacognosy, 12th Edn, ELBS Publication, Baillier
Tindall, East Bourne, pp. 344-352, 539, 1996.
9.
Murugesan
T, Mandal SC, Bhakta T, Das J, Pal M, Saha BP. Evaluation of anti-pyretic
potential of Jussiaea suffrutucosa
Linn. Extract in rats. Phytomedicine, 7: 231-234, 2000.
10.
Chattopadhyay
D, Arunachalam G, Mandal AB, Mandal SC. Evaluation of antipyretic activity of
leaf extracts of Mallotus peltatus (Geist) Muell. Arg. var acuminatus:
A Folk medicine. Phytomedicine, 9:
727-730, 2002.
11.
Guyton,
AC.; Hall, JE., Textbook of Medical Physiology. 9th Edn.
W.B.Saunders Company, Philadelphia, pp. 920-922, 1998.
12.
Kelm MA,
Nair MG,
Strasburg
GM, DeWitt DL. Antioxidant
and cyclooxygenase inhibitory phenolic compounds from Ocimum sanctum Linn. Phytomedicine.
7(1):7-13, 2000.
13.
Kinsella
JE, Lokesh B, Broughton S, Whelan J. Dietary polyunsaturated fatty acid and
eicosanoids; potential effects on the modulation of inflammatory and immuned
cells: an overview. Nutrition, 6: 24-44, 1990.
14.
Gupta MB, Nath R,
Srivastava N, Shankar K, Kishor K, Bhargava KP. Antiinflammatory and
antipyretic activities of b-sitosterol. Internat J Immunopharmacol, 18: 693-700, 1996.
15.
Bouic PJD. Plant sterols and sterolins:
a review of their immune-modulating properties. Altern Med Rev, 4:170-177, 1999.
16.
Wagner,
H., Immunostimulants and adaptogens from plants. Amason JT, Mata R, Romeo JT
(eds), Phytochemistry of Medicinal Plants. Plenum Press, New York, pp.
1-18, 1995.
17.
Chattopadhyay
D, Arunachalam G, Sur TK, Bhattacharya SK, Mandal AB. Analgesic and
Antiinflammatory Activity of Alstonia
macrophylla and Mallotus peltatus leaf extracts: Two
popular Ethnomedicines of Onge, A Nigrito Tribes of Little Andaman. Oriental
Pharmacy and Experimental Medicine
5(2) in press, 2005.
18.
Liu J.
Pharmacology of oleanolic and ursolic acid. J Ethnopharmacol, 49:57-68,
1995.
19.
Chattopadhyay
D, Arunachalam G, Mandal AB, Sur TK, Mandal SC, Bhattacharya SK. Antimicrobial
and antiinflammatory activity of folklore Mallotus
peltatus leaf extract. J
Ethnopharmacol, 82,
229-237, 2002.
20.
Tsuruga T, Chun Y, Ebizuka Y, Sankawa U. Biologically active
constituents of Melaleuca leucadendron;
inhibitors of induced histamine release from rat mast cells. Chem Pharmacol Bull,
39: 3276-3278, 1991.
21.
Kosuge T,
Yokota M, Sugiyama K, Mure T, Yamazawa H, Yamamoto T. Studies on bioactive
substances in crude drugs used for arthritic disease in trational Chinese
medicine. III. Isolation and identification of anti-inflammatory and analgesic
principles from the whole herb of Pyrola rotundifolia L. Chem
Pharmaceut Bull, 33: 5355-5357, 1985.
22.
Ying QL,
Rinehart AR, Simon SR, Cheronis JC. Inhibition of human leucocyte elastase by
ursolic acid. Biochemical J, 277: 521-526, 1991.
23.
Najid A, Simon A,
Cook J, Chable-Rabinobitch H, Delage C, Chulia A, Riguad M. Characterization of ursolic acid as a
lipoxygenase and cyclooxygenase inhibitor using macrophages, platelets and
differentiated HL 60 leukemic cells. FEBS, 229: 213-217, 1992.
24.
Ringbom
T, Segura L, Noreem Y, Perera P, Bohlin L. Ursolic acid from Plantago major,
a selective inhibition of cyclooxygenase-2 catalyzed prostaglandin
biosynthesis. J Nat Prod, 61: 1212-1215, 1998.
25.
Wang BH,
Polya GM. Selective inhibition of cyclic AMP-Dependent protein kinase by
amphiliphilic triterpenoids and related compounds. Phytochemistry, 41:
55-63, 1996.
26. Karin M, Smeal T. Control of transcription factors by signal transduction pathways: the beginning of the end. Trends Biochem Sciences, 17: 418-422, 1992.
Published by the Canadian Society for Pharmaceutical Sciences.
Copyright © 1998 by the Canadian Society for Pharmaceutical Sciences.
CSPS Home | JPPS Home | Search | Subscribe to JPPS