J Pharm Pharmaceut Sci (www.cspscanada.org) 8(2):199-206, 2005
The effects of l-arginine, d-arginine, l-name and methylene blue on channa striatus-induced peripheral antinociception in mice.
Zainul Amiruddin Zakaria*ab, Mohd. Roslan Sulaimanac, Muhammad Nazrul Somchitac, Abdul Manan Mat Jaisac, Daud Israf Alic
aDepartment of Biomedical Sciences, Faculty of Medicine and Health Sciences, Universiti Putra Malaysia, UPM Serdang, Selangor
bSchool of Biotechnology and Life Sciences, Universiti Industri Selangor, Jalan Zirkon A 7/A, Seksyen 7, Shah Alam, Selangor, Malaysia
aDepartment of Biomedical Sciences, Faculty of Medicine and Health Sciences, Universiti Putra Malaysia, 43400 UPM Serdang, Selangor
cLaboratory of Pharmaceutical and Neutriceutical, Institute of BioScience, Universiti Putra Malaysia, 43400 UPM Serdang, Selangor
cLaboratory of Pharmaceutical and Neutriceutical, Institute of BioScience, Universiti Putra Malaysia, 43400 UPM Serdang, Selangor.
Received 16 March 2005, Revised 28 March 2005, Accepted 29 March 2005, Published 3 August 2005
Abstract
PURPOSE: To determine the involvement of nitric oxide/cyclic guanosine monophosphate (NO/cGMP) pathway in aqueous supernatant of haruan (Channa striatus) fillet (ASH) antinociception using the acetic acid-induced abdominal constriction test. METHODS: The ASH was prepared by soaking fresh haruan fillet in chloroform:methanol (CM) (2/1 (v/v)) for 72 h followed by evaporation of the upper layer supernatant to remove any solvent residues. The supernatant was then subjected to a freeze-drying process (48 h) followed by doses preparation. RESULTS: Subcutaneous (SC) administration of ASH alone (0.170, 0.426 and 1.704 mg/kg) exhibited a dose-dependent antinociception. On the other hand, 20 mg/kg (SC) of L-arginine and MB exhibited a significant nociception and antinociception, while D-arginine and L-NAME did not produce any effect at all. Pre-treatment with L-arginine was found to significantly reverse the three respective doses of ASH antinociception; pre-treatment with D-arginine did not produce any significant change in the ASH activity; pre-treatment with L-NAME only significantly increased the 0.170 and 0.426 mg/kg ASH antinociception; and pre-treatment with MB significantly enhanced the respective doses of ASH antinociception, respectively. Furthermore, co-treatment with L-NAME significantly enhanced the L-arginine reversal effect on 0.426 mg/kg ASH antinociception. In addition, MB significantly reversed the L-arginine nociception on 0.426 mg/kg ASH. CONCLUSIONS: These finding suggest ASH antinociception involves the nitric oxide (NO)/cyclic guanosine monophosphate (cGMP) pathway. The presence of NO was found to reverse ASH antinociceptive activity while blocking of cGMP system enhanced it.
Introduction
Channa striatus or snakehead fish, known locally to the Malays as haruan, is a freshwater, air-breather and carnivorous fish indigenous to many tropical and subtropical countries including Malaysia [1]. Traditionally, this fish is believed to promote wound healing and alleviates post-operative pain and discomfort [2]. Mat Jais et al. [3], in addition, have reported on its potential as an antinociceptive agent in which they found that the fillet and mucus extracts of haruan exhibited a concentration-dependent activity. In addition, Mat Jais et al. [3] also suggested that both extracts act mainly through the peripheral nervous system rather than central nervous system since they only showed their effects on their own when assessed by the abdominal constriction test but not the tail-flick test. Furthermore, the mucus antinociception was reported to be stable under extreme temperature and pH and did not involve an opioid receptor mechanism [4].
Nitric oxide (NO) has been reported to activate soluble guanylate cyclase and to increase the intracellular cGMP, which may then modulate a great number of physiological functions, such as circulatory vessel dilation [5] and immune system [6]. Furthermore, NO has also been reported to participate in both central and peripheral nociceptive processing by acting as a pronociceptive as well as an antinociceptive agent at supraspinal and peripheral sites [7;8]. However, precise mechanisms underlying the opposing effect of NO on nociception are yet unknown. A series of morphologic [9], physiologic [10] and pharmacologic [11] studies suggest that NO participates in some way in the process of nociception. The importance of cyclic 3':5'-cyclic guanosine monophosphate (cGMP) pathway in mediating the nociceptive process within the spinal [12] and supraspinal [13] level has also been proven. Based on the above findings on the importance of NO/cGMP pathway in nociceptive mechanism, our main objective in the present study was to evaluate the involvement of NO/cGMP pathway in modulating the aqueous supernatant of haruan fillet (ASH) antinociception.
Methodology
Preparation of Fresh Fillet of Haruan and ASH
Preparation of fresh haruan fillet was carried out according to the method described by Mat Jais et al. [3]. The ASH was prepared using the chloroform:methanol (CM) (2:1; v:v) system [14] with slight modifications as described by Zakaria et al. [15]. The fillet obtained was directly mixed with CM in the ratio of 1:2 (w:v) and left overnight for 24 hrs before further processes were carried out. The sample was then filtered and the supernatant left for another 30 min to settle down. At the end of 30 min, two layers could be seen clearly in the supernatant. The upper layer, which is an aqueous layer was collected and used for this study while the lower layer, which is the chloroform:methanol layer was stored at 4oC until used. The aqueous layer obtained was then subjected to the evaporation process to remove the methanol residues followed by the freeze-drying process to determine the exact amount of dried sample for doses preparation. The dried sample obtained was then prepared in three different doses, 0.107, 0.426 and 1.074 mg/kg, which are approximately equal to the concentration/strength of 10, 25 and 100%, as described by Mat Jais et al. [3], of evaporated aqueous layer not subjected to freeze-drying process.
Preparation of Drugs
Acetylsalicylic acid (ASA) (Bayer, Singapore), used as positive control group, was prepared in the dose of 10 mg/kg while L-arginine (Sigma, U.S.A.), D-arginine (Sigma, U.S.A.), N G -Nitro-L-Arginine methyl esters (L-NAME; Sigma, U.S.A.) and methylene blue (MB; Sigma, U.S.A.) were prepared in the dose of 20 mg/kg [16;17], by dissolving them in distilled water (DH 2 O).
Experimental Animals
Male Balb-C mice (25-30 g; 5-7 weeks old) were used in this study to minimize the effect of hormonal changes within the mice body and kept under room temperature (27±2oC; 70-80% humidity; 12 h light/darkness cycle) in the Animal Holding Unit (UPM) for at least 48 h before used. Food and water were supplied ad libitum up to the beginning of the experiments. At all times the experimental procedures were carried out in strict compliance with the Animal Ethics Committee rules and regulation followed by the University and the ethical guidelines for investigations of experimental pain in conscious animals [18]. All experiments were conducted between 09.30 and 12.30 h to minimize the effects of environmental changes. All mice were divided into twenty-five groups of ten mice each (n = 10). Five groups were pre-treated with DH 2 O followed 5 min later by treatment with DH 2 O, ASA (10 mg/kg), or ASH (0.107, 0.426 and 1.074 mg/kg), respectively. The remaining 20 groups were pre-treated with 20 mg/kg L-arginine, D-arginine, L-NAME or MB followed 5 min later by treatment with DH 2 O, ASA, or ASH (0.107, 0.426 and 1.074 mg/kg), respectively. 30 min after administration of the respective DH 2 O, ASA, or ASH, the mice were injected with 0.6% acetic acid intraperitoneally (IP) [3; 4]. All drugs and solutions were administered subcutaneously (SC) in the volume of 10 ml/kg.
Antinociceptive Assay
The abdominal constriction test [19] was used to determine the effects of L-arginine, D-arginine, L-NAME and MB on ASH-induced antinociceptive activity. Briefly, the acetic acid (J.T. Baker, U.S.A.), prepared as 0.6% (v:v) solution in DH2O and used to induce pain in mice peritoneal cavity, was administered IP (10 ml/kg of mice) 30 min after the SC administration of respective DH 2 O, ASA or ASH. The abdominal constriction or writhing response resulting from the injection of acetic acid consisting of a contraction of the abdominal together with a stretching of the hind limbs [20]. The number of abdominal constrictions was counted cumulatively over the period of 25 min, 5 min following the acetic acid administration. Antinociception was calculated, using a formula described by Dambisya and Lee [19], as the percentage inhibition of abdominal constrictions (percentage of inhibitory level) using the formula ([Saline control group mean - test group mean]/Saline control group mean) X 100% and presented in the form of histogram.
Statistical Analysis
The results are presented as Mean ± Standard Error of Mean (S.E.M.). The one-way ANOVA test followed by Duncan post-test was used to analyze and compare the data, with P<0.05 as the limit of significance.
ResultS
Effects of L-arginine, D-arginine, L-NAME and MB on ASH Antinociception
The results obtained for groups of mice treated with DH 2 O, ASA or ASH (0.107, 0.426 and 1.074 mg/kg) after pre-treatment with DH 2 O showed a significant dose-dependent antinociception and are presented in all following figures for comparison against the drugs pre-treated groups.
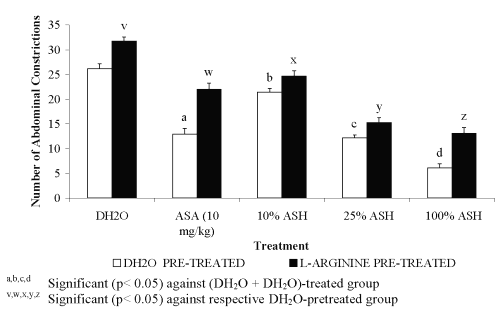
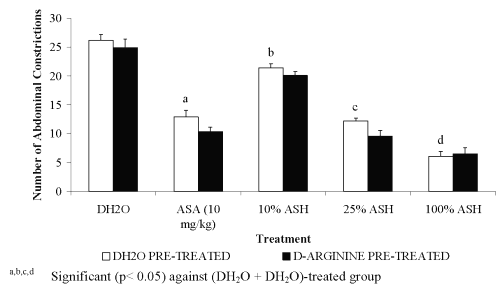
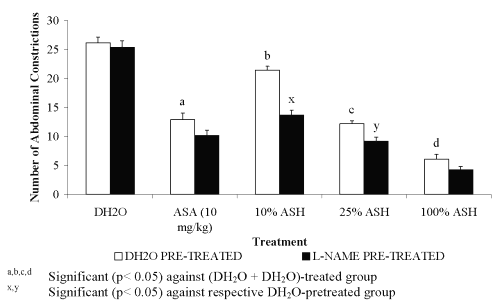
For pre-treatment with 20 mg/kg L-arginine, all groups showed significant decreased in the percentage of antinociception when compared to the DH 2 O pre-treated groups (Figure 1). The negative results in the percentage of antinociception obtained indicated the ability of L-arginine to reverse the neutral effect produced by DH 2 O, which devoid of nociceptive or antinociceptive activity, and antinociceptive effect produced by ASA or ASH. Pre-treatment with 20 mg/kg D-arginine did not significantly alter the percentage of antinociception when compared to the DH 2 O pre-treated groups (Figure 2). Except for DH 2 O-, ASA- and 1.074 mg/kg ASH-treated groups, pre-treatment with 20 mg/kg of L-NAME was found to significantly enhance the antinociceptive activity of 0.107 and 0.426 mg/kg ASH, respectively when compared to the DH2O pre-treated groups (Figure 3).
Figure 1: Effect of D-arginine (20mg/kg) on ASA and ASH Antinociceptive Activity.
Figure 2: Effect of L-arginine (20mg/kg) on ASA and ASH Antinociceptive Activity.
Figure 3: Effect of L-NAME (20mg/kg) on ASA and ASH Antinociceptive Activity.
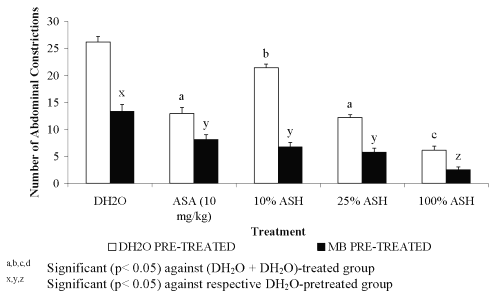
Pre-treatment with 20 mg/kg of MB was found to cause highly significant increased in the percentage of antinociception of ASA and the doses of ASH. Furthermore, MB was also found to cause remarkable antinociception on its own indicating its potential as antinociceptive agent when compared to the DH 2 O pre-treated groups (Figure 4).
Figure 4: Effect of MB (20mg/kg) on ASA and ASH Antinociceptive Activity.
Effect of Combination of L-arginine with L-NAME or MB on ASH Antinociceptive Activity
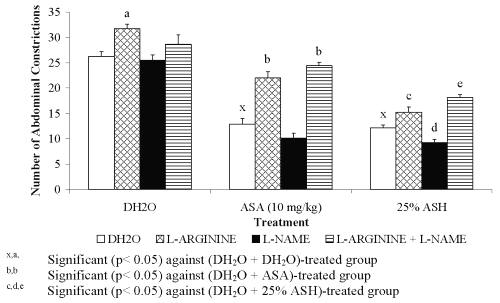
The effect of combining L-arginine with L-NAME or MB on the antinociceptive activity of 0.426 mg/kg ASH was also studied and the data were presented in Figure 5 and Figure 6, respectively.
Figure 5: Effect L-arginine, L-NAME or its combination (L-arginine + MB) on ASA and ASH Antinociceptive Activity.
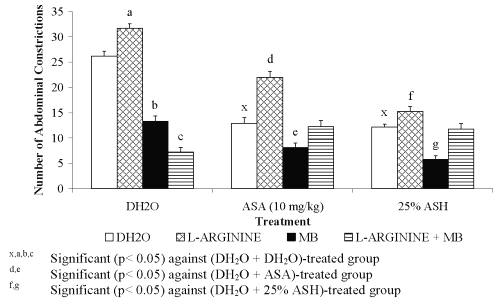
Figure 6: Effect L-arginine, MB or its combination (L-arginine + MB) on ASA and ASH Antinociceptive Activity.
0.426 mg/kg ASH was chosen for this study because it was found to produce approximately 50% antinociception. Combination of L-arginine and L-NAME was found to cause insignificant change in the percentage of antinociception of DH 2 O-treated group (L-arginine + L-NAME + DH 2 O) when compared to the (DH 2 O + DH 2 O)- and (L-arginine + DH 2 O)-treated groups, respectively. This finding seems to suggest the slight and insignificant ability of L-NAME at 20 mg/kg to reverse the nociceptive effect of L-arginine. The respective combination of L-arginine and L-NAME, on the other hand, was found to produce remarkable decrease ( P <0.001) in the percentage of antinociception of ASA-treated group (L-arginine + L-NAME + ASA) when compared to the (DH 2 O + ASA)-treated group. This finding, however, is not seen when compared to the (L-arginine + ASA)-treated group. The observed data seem to suggest the failure of L-NAME at 20 mg/kg to reverse or enhance the nociceptive effect of L- arginine on ASA antinociception. Interestingly, the combination of L-arginine and L-NAME was found to significantly decreased the percentage of antinociception of 0.426 mg/kg ASH (L-arginine + L-NAME + 0.426 mg/kg ASH) when compared to both the (DH 2 O + 0.426 mg/kg ASH)- and (L-arginine + 0.426 mg/kg ASH)-treated groups, respectively. This seems to suggest that L-NAME at 20 mg/kg enhanced the L-arginine-induced reversal of 25% concentration ASH antinociception.
Combination of L-arginine and MB, on the other hand, was found to produce highly significant increased in the percentage of antinociception of DH 2 O-treated group (L-arginine + MB + DH 2 O) when compared to the (DH 2 O + DH 2 O)- and (L-arginine + DH 2 O)-treated groups, respectively. The data obtained suggested the complete reversal of L-arginine-induced nociception by MB. Interestingly, the respective combination of L-arginine and MB was found to produce similar pattern of data when administered with ASA or 0.426 mg/kg ASH. The (L-arginine + MB + ASA)-treated group was found to produce a significant increased in the percentage of antinociception when compared to the (L-arginine+ ASA)- but not the (DH2O + ASA)-treated group, respectively. Similarly, the (L-arginine + MB + 0.426 mg/kg ASH)-treated group was found to produce a significant increased in the percentage of antinociception when compared to the (L-arginine+ 0.426 mg/kg ASH)- but not the (DH2O + 0.426 mg/kg ASH)-treated group, respectively. From the data obtained, MB was also found to reverse the L-arginine-induced reversal of 0.426 mg/kg ASH antinociceptive activity.
Discussion
NO, a major player in physiological functions as in the impulse transmission in the central and peripheral nervous system [21], protection against tumors and infection [22] and dilation of blood vessels [23], has been reported to be involved in the mechanism of nociception by acting as a pronociceptive as well as an antinociceptive agent at the supraspinal and peripheral sites [7;8]. Studies suggested that NO may induce pronociceptive effects at low concentrations and antinociceptive ones at higher concentrations [24;25]. At the supraspinal level NO appeared to play a dual role in the nociceptive processing depending on which pathways were activated [13]. The activation of the kyotorphin-Met-enkephalin pathway was described to lead to antinociception, but activation of the NO-cGMP pathway resulted in hyperalgesia [13]. The peripheral mechanism of nociceptive transmission involving NO appears more complex since both inhibitory and promotive actions of NO have been reported. Topically administered L-arginine exhibited antinociceptive activity in rats with carrageenin-induced hyperalgesia, the effect being blocked by NOS and guanylate cyclase inhibitors [26]. Contrary to the above finding, L-arginine was found to induce nociceptive activity in inflammatory responses elicited by substance P, carrageenin and bradykinin, the effect being suppressed by L-NAME [27]. These findings confirm nociceptive and antinociceptive roles of the NO-cGMP pathway in the peripheral tissues. According to Kawabata et al. [24], the dual role played by peripheral NO in nociceptive modulation, inducing either nociception or antinociception, depends on its tissue level. This suggestion was based on the authors' observations that L-arginine enhanced the second- but not the first-phase nociception at a lower dose but produced antinociception at a higher dose. Moreover, L-NAME at a lower dose exhibited antinociceptive activity in the second-phase and reduced the increase in second-phase nociception elicited by low dose of L-arginine. In addition, the second-phase of nociception decrease, induced by a higher dose of L-arginine, was markedly reversed by a low dose of L-NAME.
In the present study, 20 mg/kg L-arginine, a precursor of NO production, was found to cause hyperalgesia when given alone (SC) and reversed the antinociceptive activity of ASA and ASH when administered together. This finding is in parallel with a previous report by Abacolu et al. [16] that L-arginine, at the same dose, exhibited the hyperalgesic activity when administered alone (SC) when assessed using the p-benzoquinone-induced abdominal constriction test. The ability of L-arginine to reverse the ASA antinociception was described in a report by Björkman et al. [28] that L-arginine is capable of reversing the acetaminophen antinociception in substance P- or NMDA-induced hyperlalgesia. The L-arginine-induced reversal of ASH antinociceptive activity was also consistent with an earlier finding by Brignola et al. [29] that L-arginine suppressed the morphine-induced antinociception when assessed by the acetic acid-induced abdominal constriction test. According to Kawabata et al. [24] the nociceptive effect elicited by L-arginine may represent direct actions on nociceptors and would not be influenced by NO production as suggested by Abacolu et al. [30].
Pre-treatment with 20 mg/kg D-arginine was found to produce results that are similar to previous findings by Brignola et al. [29] and Abacolu et al. [16] in which it neither exhibited nociceptive nor antinociceptive activity when given alone or co-administered with any antinociceptive agents. The compound was also found to be inactive when administered alone or pre-treated with ASA and ASH, respectively.
Pre-treatment of 20 mg/kg L-NAME, an inhibitor of NOS, alone was found to give results in keeping with a report by Jain et al. [17]. The authors reported on the failure of L-NAME alone, at the respective dosage, to affect the nociceptive threshold when assessed against the acetic acid-induced abdominal constriction test. The results of Jain et al. [17] and our results in the present study disagree with findings reported by Abacolu et al. [16]. The latter reported on the antinociceptive effect of L-NAME at doses of 18.75, 37.5, 75 and 150 mg/kg, respectively. This suggests that the acetic acid-induced algesia is not influenced by decrease in endogenous NO within the peripheral tissue. In addition, L-NAME was also found to be ineffective against the antinociceptive effect of ASA, which is in contrast with the report published by Bujalska and Gumulka [31]. The latter had previously reported on the ability of LG-nitro-L-arginine (L-NO-ARG), a NOS inhibitor, to enhance the antinociceptive activity of the lower subceiling doses of acetaminophen. The present finding seems to support previous statement made by Ing et al. [32] who reported that the ultimate effects of NO are dependent on dosage levels used and the rate and timing of NO release. It is suggested that the level of L-NAME dosage used in the present study does not have enough ability to affect the ASA-induced antinociception or produce enough reduction of NO leading to its failure to change the ASA-induced antinociceptive activity. ASA-induced antinociception not affected by decrease in NO concentration within the peripheral tissue needs to be considered. ASA activity is known to involve a cylco-oxygenase pathway, which in turn does not involve NO activity, which explains the failure of L-NAME to change the ASA activity. Furthermore, the ability of 20 mg/kg L-NAME to increase the antinociceptive activity of both the 0.107 and 0.426 mg/kg ASH was found to be in contrast with the report made by Jain et al. [33] that L-NAME is capable of reversing the sildenafil antinociceptive activity. Moreover, our finding on the failure of L-NAME to increase the antinociceptive effect of 1.074 mg/kg ASH is in keeping with Bujalska and Gumulka [31] who reported on the failure of L-NO-ARG to affect the acetaminophen antinociception at supramaximal doses. It is suggested that L-NAME's ability to enhance ASH antinociceptive activity is due to its ability to increase vascular permeability [26] induced by acetic acid. According to Kawabata et al. [24], such effects of L-NAME may result in antinociception especially in chemically mediated nociception models.
Further studies using MB, an inhibitor of cGMP pathway, revealed the ability of MB to act as antinociceptive agent on its own, which is concomitant with the report published by Abacolu et al. [16], and to enhance other antinociceptive agents (ASH and ASA) activity, respectively. Our finding on the enhancement of ASH antinociception by MB indicates the important role of cGMP pathway in pain impulse transmission. Since cGMP pathway is important in the pain mechanism, blocking of this pathway leads to reduction in pain sensation, explains the remarkable improvement of ASH and ASA antinociceptive activity.
In conclusion, our studies indicate that ASH antinociception is influenced by the presence or absence of NO and that this activity may involve the cGMP pathway.
Acknowledgments
This study was supported by the Research Grant of Universiti Industri Selangor, Malaysia. (Project Code Number: 03013; Project Vote Number: 3090103013). The authors wish to thank Prof. Dr. Elizabeth George and Mrs. Fatimah Corazon Abdullah for their skilful help with the preparation of this manuscript.
References
Mohsin AK, Ambak MA. (1983). Freshwater fishes of Peninsula Malaysia. Universiti Pertanian Malaysia Press. Serdang, Malaysia, 157 – 161.
Mat Jais AM, McCullock R, Croft K. (1994). Fatty acid and amino acid composition in haruan as a potential role in wound healing. General Pharmacology 25: 947 – 950.
Mat Jais, A.M., Dambisya, Y.M. and Lee, T.L. (1997). Antinociceptive Activity of Channa striatus (Haruan) Extracts in Mice. Journal of Ethnopharmacology 57: 125 – 130.
Dambisya, Y.M., Lee, T.L., Sathivulu, V.c and Mat Jais, A.M.c(1999). Influence of Temperature, pH and Naloxone on the Antinociceptive Activity of Channa striatus (Haruan) Extracts in Mice. Journal of Ethnopharmacology 66: 181 – 186.
Stoclet, J., Muller, B., Gyorgy, K., Andriantsiothaina, R. and Kleschyov, A. (1999). The Inducible Nitric Oxide Synthase In Vascular and Cardiac Tissue. European Journal of Pharmacology 375: 139 – 155.
Jungi, T., Adler, H., Thöny, M., Krampe, M. and Peterhans, E. (1996). Inducible Nitric Oxide Synthase of Macrophages: Present Knowledge and Evidence for Species-specific Regulation. Veterinary Immunology and Immunopathology 54: 323 – 330.
Ferreira, S.H., Duarte, I.D.G. and Lorenzetti, B.B. (1991). The Molecular Mechanism of Action of Peripheral Morphine Analgesia: Stimulation of the cGMP System via Nitric Oxide Release. European Journal of Pharmacology 201: 121 – 122.
Machelska, H., Labuz, D., Przewlocki, R. and Przewlocka, B. (1997). Inhibition of Nitric Oxide Synthase Enhances Antinociception Mediated by Mu, Delta and Kappa Opioid Receptors in Acute and Prolonged Pain in the Rat Spinal Cord. Journal of Pharmacology and Experimental Therapeutics 282: 977 – 984.
Herdegen, T., Rudiger, S., Mayer, B., Bravo, R. and Zimmermann, M. (1994). Expression of Nitric Oxide Synthase and Colocalisation with Jun, Fos and Krox Transcription Factors in Spinal Cord Neurons Following Noxious Stimulation of the Rat Hindpaw. Molecular Brain Research 22: 245 – 258.
Garthwaite, J. and Boulton, C.L. (1995). Nitric Oxide Signaling in the Central Nervous System. Annual Review of Physiology 57: 683 – 706.
Moncada, S., Palmer, R.M.J. and Higgs, E.A. (1989). Biosynthesis of Nitric Oxide from L-arginine: A Pathway for the Regulation of Cell Function and Communication. Biochemical Pharmacology 38: 1709 – 1715.
Morris, R., Southam, E. Braid, D.J. and Garthwaite, J. (1992). Nitric Oxide May Act as a Messenger Between Dorsal Root Ganglia Neurones and Their Satellite Cells. Neuroscience Letter 137: 29 – 32.
Kawabata, A., Umeda, N. and Takagi, H. (1993). L-Arginine Exerts a Dual Role in Nociceptive Processing in the Brain: Involvement of the Kyotorphin-met-enkephalin Pathway and NO-cyclic GMP Pathway. British Journal of Pharmacology 109: 73 – 79.
Benkendorff, K., Bremner, J.B. and Davis, A.R. (2001). Indole Derivatives from the Egg Masses of Muricid Molluscs. Molecules 6: 70 – 78.
Zakaria, Z.A. (2001). Some Physical and Chemical Properties of the Bioactive Components Responsible for Antinociceptive Activity of Haruan (Channa striatus) Extracts. MSc. Thesis, Universiti Putra Malaysia, Malaysia.
Abacıoğlu, N., Tunçtan, B., Akbulut, E. and Çakıcı, İ. (2000). Participation of the Components of L-arginine/Nitric Oxide/cGMP Cascade By Chemically-induced Abdominal Constriction in the Mouse. Life Sciences 67: 1127 – 1137.
Jain, N.K. and Kulkarni, S.K. (1999). L-NAME, a Nitric Oxide Synthase Inhibitor, Modulates Cholinergic Antinociception. Methods and Findings in Experimental and Clinical Pharmacology 21: 161 – 165.
Zimmermann, M. (1983). Ethical Guidelines for Investigations of Experimental Pain in Conscious Animals. Pain 16: 109 – 110.
Dambisya, Y.M. and Lee, T.L. (1995). Effects of L-NAME, L-NMMA and L-arginine on the Antinociceptive Effects of Morphine in Mice. Methods and Findings in Experimental and Clinical Pharmacology 17: 577 – 582.
Correa, C.R., Kyle, D.J., Chakraverty, S. and Calixto, J.B. (1996). Antinociceptive Profile of the Pseudopeptide B2 Bradykinin Receptor Antagonist NPC 18688 in Mice. British Journal of Pharmacology 117: 552 – 558.
Garthwaite, J. (1991). Glutamate, Nitric Oxide and Cell-cell Signaling in the Nervous System. Trends in Neurosciences 14: 60 – 67.
Kilbourn, R.G., Traber, D.L. and Szabo, C. (1997). Nitric Oxide and Shock. Disease-A-Month 43: 277 – 348.
Johnson, M.L. and Billiar, T. (1998). Roles of Nitric Oxide in Surgical Infection and Septics. World Journal of Surgery 22: 187 – 196.
Kawabata, A., Manabe, S., Manabe, Y. and Takagi, H. (1994). Effect of Topical Administration of L-arginine on Formalin-induced Nociception in the Mouse: A Dual Role of Peripherally Formed Nitric Oxide in Pain Modulation. British Journal of Pharmacology 112: 547 – 550.
Meller, S.T., Dykstra, C. and Gebhart, G.F. (1992a). Production of Endogenous Nitric Oxide and Activation of Soluble Guanylate Cyclase are Required for N-methyl-D-aspartate-produced Facilitation of the Nociceptive Tail-flick Reflex. European Journal of Pharmacology 214: 93 – 96.
Duarte, I., Lorenzetti, B., Ferreira, S. (1990). Peripheral Analgesia and Activation of the Nitric Oxide-cyclic GMP Pathway. European Journal of Pharmacology 186: 289 – 293.
Ialenti, A., Ianaro, A., Moncada, S. and Di Rosa, M. (1992). Modulation of Acute Inflammation by Endogenous Nitric Oxide. European Journal of Pharmacology 211: 177 – 182.
Björkman, R., Hallman, K.M., Hedner, J., Hedner, T. and Henning, M. (1994). Acetaminophen Blocks Spinal Hyperalgesia Induced by NMDA and Substance P. Pain 57: 259 – 264.
Brignola, G., Calignano, A. and Di Rosa, M. (1994). Modulation of Morphine Antinociception in the Mouse by Endogenous Nitric Oxide. British Journal of Pharmacology 113: 1372 – 1376.
Abacıoğlu, N., Tunçtan, B., Çakıcı, İ., Akbulut, E., Uludağ, O., Kanzık, İ. (2001). The Role of L-arginine/Nitric Oxide Pathway in the Antinociceptive Activity of Pyridoxine in Mouse. Arzneimittelforschung 51: 832 – 838.
Bujalska, M. and Gumulka, W.S. (2001). Effects of Cyclooxygenase and NO Synthase Inhibitors on Antinociceptive Action of Acetaminophen. Polish Journal of Pharmacology 53: 341 – 350.
Ing, D., Zang, J., Dzau, V., Webster, K. and Bishopric, N. (1999). Modulation of Cytokine-induced Cardiac Myoctye Apoptosis by Nitric Oxide, Bak, and Bcl-x. Circulation Research 84: 21 – 33.
Jain, N.K., Patil, C.S., Singh, A. and Kulkarni, S.K. (2001). Sildenafil-induced Peripheral Analgesia and Activation of the Nitric Oxide – cyclic GMP Pathway. Brain Research 909: 170 – 178.
Corresponding Author: Zainul Amiruddin Zakaria, School of Biotechnology and Life Sciences, Universiti Industri Selangor, Jalan Zirkon A 7/A, Seksyen 7, 40000 Shah Alam, Selangor, Malaysia. shaza1874@hotmail.com; shaza8174@yahoo.com
Published by the Canadian Society for Pharmaceutical Sciences.
Copyright © 1998 by the Canadian Society for Pharmaceutical Sciences.
http://www.cspscanada.org