J Pharm Pharmaceut Sci (www.cspscanada.org) 8(2):207-216, 2005
Influence of packaging material on the liquid stability of interferon-α2b
Llamil Ruiz1, Nuria Reyes1, Kethia Aroche1, Vivian Tolosa2, Vladimir Simanca2, Teresa Rodríguez2 and Eugenio Hardy1
Received December 6, 2004, Revised March 7, 2005, Accepted May 18, 2005, Published August 4, 2005Abstract. Purpose: In this article we studied the effect of the packaging material on the liquid stability of interferon alpha 2b (rhIFN-a2b). Methods: The compatibility of this cytokine with type I borosilicate glass ampoules was evaluated by ELISA and RP-HPLC, at 4ºC and after heat sealing. Additionally, the influence of protein concentration (3 and 10 MIU/ml), buffer species (sodium phosphate, sodium citrate and sodium citrate-phosphate) and additives (polysorbate 80 and EDTA Na2 x 2H2O) were studied in samples with and without contact with chlorobutyl stoppers by RP-HPLC. Results: The compatibility of this cytokine in sodium phosphate buffer, with type I borosilicate glass ampoules showed a significant adsorption at the lowest concentration. This influence was eliminated with a polysorbate 80/benzyl alcohol-based vehicle. The effect of the heat sealing of ampoules on the stability of rhIFN-a2b showed two degradation peaks when a volume of 1 ml was dispensed. However, with a lower (0.5 ml) volume, the degradation was not detected. On the other hand, samples in contact with chlorobutyl stoppers increased the apparent degradation rate constant in the range of 6.74 ± 0.38 to 46.34 ± 3.11 x 103 day-1. This effect significantly decreased in about 1.2- and 1.1-fold when sodium citrate or sodium citrate-phosphate buffers, respectively, were evaluated. Results from the evaluation of EDTA Na2 x 2H2O or polysorbate 80 showed a similar behavior. These additives reduced the apparent degradation rate constant in the range of 2.01 ± 0.14 to 25.51 ± 3.57 x 103 day-1. Conclusions: The adsorption of the cytokine to type I borosilicate glass ampoules was eliminated with a polysorbate 80/benzyl alcohol-based vehicle, and the deleterious effect of the heat sealing decreased with a lower (0.5 ml) volume. Experimental data indicated that the contact with chlorobutyl stoppers accelerates the degradation of rhIFN-a2b. However, protein concentration, buffer species and pharmaceutical excipients can modulate this effect.
Introduction
Interferon alpha 2b (rhIFN-a2b) is a cytokine with a wide use in viral, neoplasic and immunological diseases due to its strongly verified biological properties (1; 2). Many factors can affect the liquid stability of this and other proteins, inducing a great number of physical and chemical degradation pathways (3). One of such factors is the packaging material (3; 4). Several authors have studied the adsorption of proteins to surfaces of untreated glass, siliconized glass, polyester, polypropylene, nylon, silicone rubber and cellulose acetate (3). These studies have demonstrated the instability of proteins at these surfaces, probably due to the adsorption-induced protein denaturation (3).
The general accepted mechanism explaining this effect is based on the high surface tension at the liquid/packaging material interfaces. This tension usually leads proteins to the loss of their tridimensional structure, and increases the probability of adsorption, aggregation, denaturation and other physical and chemical reactions (3). Surfactants have been frequently used to reduce this effect (5; 6). The role of detergents has been to protect proteins through the competition for the adsorption at different interfaces. Furthermore, interactions with the protein surface have been described (3). In this sense, detergents may cover the hydrophobic sites of proteins reducing the occurrence of aggregation or act as “chaperonins” to catalyze the refolding of partially unfolded molecules (6).
Most of the biopharmaceutical drugs are prepared in borosilicate glass vials sealed with chlorobutyl stoppers. The effect of these materials on the stability of proteins must also be studied. Earle et al. evaluated the increment of the residual moisture due to chlorobutyl stoppers, on the stability of Haemophilus influenzae conjugate vaccine (7). However, the gum-stoppers induced degradation has not been clearly studied in protein solutions. Herein, we evaluated the effect of borosilicate glass ampoules and chlorobutyl stoppers on the stability of rhIFN-a2b in solution.
Materials and Methods
Materials
The
Center for Genetic Engineering and Biotechnology (CIGB,
a2b in solution
rhIFN-a2b was diluted to 1.5, 3, 5, 10 and 20 million international units per milliliter (MIU/ml) in sodium phosphate buffer or in a polysorbate 80/benzyl alcohol-based vehicle, and 0.5 ml was dispensed in borosilicate glass ampoules (Bormioli Rocco, Parma, Italy). Each vial was sealed with parafilm and stored at 4ºC for 24 or 120 h. The compatibility of glass vials with rhIFN-a2b was estimated by determining the concentration of ELISA-quantified rhIFN-a2b present at 24 or 120 h of storage, compared to the initial concentration.
rhIFN-a2b was also diluted to 3 MIU/ml, in 50 mM sodium phosphate
buffer, pH 6 (43.8 mM sodium phosphate monobasic dihydrate, 6.2 mM sodium
phosphate dibasic anhydrous). The interferon-containing samples of 0.5 or
1 ml were dispensed into borosilicate glass ampoules (
Effect of the protein concentration on the liquid stability of rhIFN-a2b in contact with stoppers
rhIFN-a2b was diluted to 3 and 10 MIU/ml in 50 mM sodium phosphate buffer, pH 6. Samples of 1 ml were dispensed in 2R borosilicate glass vials, stored at 37ºC with or without contact with stoppers and analyzed by RP-HPLC at time zero and after 3, 6, 9, 15, 21 and 30 days. Experiments were done in triplicate.
Influence of buffer species on the liquid stability of rhIFN-a2b, in contact with stoppers
PD-10 desalting columns (Amersham Biosciences AB, Upsala, Sweden )
were used to change the buffer composition on each sample. Columns were previously
equilibrated with 50 mM sodium citrate buffer, pH 6 (6.4 mM citric acid, 43.6
mM sodium citrate dihydrate), 50 mM sodium citrate-phosphate buffer, pH 6
(19 mM citric acid, 31 mM sodium phosphate dibasic anhydrous) or 50 mM sodium
phosphate buffer, pH 6. rhIFN-
Influence of polysorbate 80 and EDTA Na2 x 2H2O on the liquid stability of rhIFN-a2b, in contact with stoppers
rhIFN-a2b was diluted to 3 MIU/ml in 50 mM sodium phosphate buffer, pH 6, and then polysorbate 80 or EDTA Na2 x 2H2O were added to the solution to a final concentration of 5 mM. Samples of 1 ml were dispensed in borosilicate glass vials, stored at 37ºC with or without contact with stoppers and analyzed by RP-HPLC at time zero and after 3, 6, 9, 15, 21 and 30 days. Experiments were done in triplicate.
Analysis of the rhIFN-a2b solutions by Enzyme Linked ImmunoSorbent Assay (ELISA)
This procedure was performed as previously described (10; 11).
Analysis of the rhIFN-a2b solutions by Reverse-Phase High-Performance Liquid Chromatography (RP-HPLC)
RP-HPLC analysis was performed on a Vydac (
Statistical analysis
The statistical significance of the experimental
data was determined by the unpaired student-t or ANOVA tests, after a comparison
of the homogeneity of variance (
Results and Discussion
a2b in solution
Adsorption is one of the physical influences that affect the stability of proteins in solution (3). This degradation route can be induced at different sites such as container/solution and air/solution interfaces. The severity of this process usually depends on the protein concentration and the protein itself, and has been inhibited with surfactants (3; 6).
We evaluated the effect of the glass ampoules on the stability of rhIFN-a2b at different concentrations. In this experiment we found that rhIFN-a2b was adsorpted only at the lowest concentration (1.5 MIU/ml) (Table 1). However, at higher concentrations, differences between results at the initial time and after 24 hours of storage were not significant (Table 1). The use of ELISA to evaluate the adsorption of the active ingredient enhanced the reliability of these results due to the ability of this technique to quantify the correctly folded interferon and recognize when degraded species coexist in the protein solution that has been analyzed (11).
Table 1. Compatibility of borosilicate glass ampoules with rhIFN-a2b diluted in sodium phosphate buffer.
| Concentration (x 106 IU/ml) |
Time (h) |
ELISA concentration (µg/ml) |
Recovery (%) |
a (0.05) |
| 1.5 |
0 |
7.61 ± 0.22 |
- |
|
| 24 |
7.13 ± 0.16 |
93.69 |
0.01 |
|
| 3 |
0 |
21.62 ± 2.23 |
- |
|
| 24 |
20.71 ± 2.57 |
95.79 |
0.07 |
|
| 5 |
0 |
30.92 ± 1.37 |
- |
|
| 24 |
29.58 ± 0.73 |
95.67 |
0.07 |
|
| 10 |
0 |
62.71 ± 4.42 |
- |
|
| 24 |
60.33 ± 2.98 |
96.21 |
0.14 |
|
| 20 |
0 |
115.38 ± 2.12 |
- |
|
| 24 |
114.91 ± 2.76 |
99.59 |
0.55 | |
| Results are expressed as mean (n=3) ± standard deviation (SD). | ||||
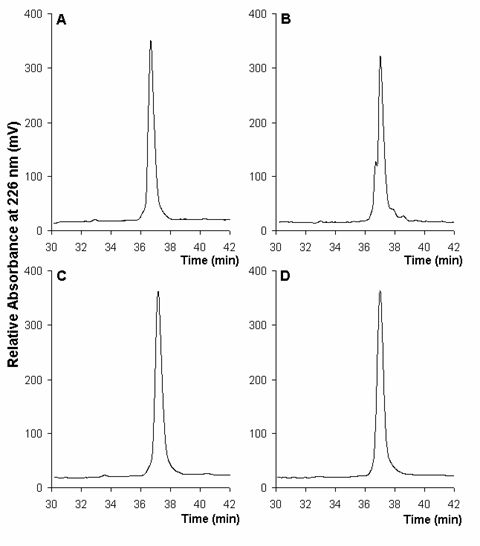
Figure 1. Effect of the heat sealing of borosilicate ampoules on the RP-HPLC profile of rhIFNa-2b. Chromatographic profiles of 1-ml samples are given in A (before sealing) and B (after sealing). Additionally, chromatographic profiles of 0.5-ml samples are given in C (before sealing) and D (after sealing).
In order to decrease the adsorption of 1.5 MIU/ml rhIFN-a2b to borosilicate glass ampoules, we evaluated the effect of a polysorbate 80/benzyl alcohol-based vehicle, at four concentrations of the cytokine. After this, we did not find statistical differences (a=0.97) between the recovery of rhIFN-a2b in the solution at the initial time and after 24 or 120 hours of storage, even at 1.5 MIU/ml (Data not shown).
The use of surfactants (e.g., polysorbate 80) has seen effective to protect proteins against adsorption (3; 5; 6). The mechanism of protection involves two main pathways (6). One such pathways is based on the specific interaction with the protein surface, covering hydrophobic sites and acting as “artificial chaperonins” to catalyzed the refolding of partially unfolded proteins (6). The second one involves the competition with the protein for the adsorption to various interfaces where chemical or physical degradation may occur (6).
Our results indicated that the stability of rhIFN-a2b at low concentrations can be compromised due to the influence of borosilicate glass ampoules; however, the use of a detergent-based vehicle may inhibit this process.
Borosilicate glass ampoules are usually sealed by heating. Although this is a very rapid process, drugs must be very stable to efficiently retain the stability during and after heating. Here, we evaluated the effect of the sealing of ampoules by heating, after dispensing 1 ml of the rhIFN-a2b solution. Results obtained by RP-HPLC showed three degradation by-products which affected the purity of the native rhIFN-a 2b peak (Fig. 1 A, B).
Although the use of the ELISA has proven the ability of this technique to quantify the correctly folded rhIFN-a2b (11), statistical analysis did not show significant differences (p=0.67) before (18.82 ± 0.74 µg/ml) and after (18.61 ± 0.81 µg/ml) sealing despite the chromatographic profile. In fact the three moieties eluting before and after the rhIFN-a2b main peak were only a very low percent of the total protein (less than 5 % each one). Additionally, the early eluting species has demonstrated to retain the same biological activity and immunoidentity than the native rhIFN-a2b (2).
Contrarily, results obtained by RP-HPLC showed one peak without degradation species, when a lower (0.5 ml) volume was dispensed into borosilicate ampoules before the heat sealing (Fig. 1 C, D). It seems that low volumes of rhIFN-a2b solutions must be dispensed into these vials if the sealing process will be based on heating.
Influence of chorobutyl stoppers on the liquid stability of rhIFN-a2b
Although the stabilization of proteins can be very variable, rhIFN-a2b has shown to be stable at different concentrations as well as in the presence of different buffers and additives (13; 14).
In this study, rhIFN-a2b showed a chromatographic profile characterized by an early eluting species and a moiety eluting just after the rhIFN-a2b main peak (Fig. 2). The first of such degradations could correspond to a Met sulfoxide by-product (2). The exact chemical identity of the later fraction remains under investigation and will be published elsewhere. These degradation products were greater induced in those samples in contact with gum stoppers and the concentration of the protein, the buffer species and the use of additives affected their intensity.
This fact clearly showed the deleterious effect of the gum stoppers on the stability of rhIFN-a2b. Two factors can be considered in order to explain this influence. The possible release of heavy metal ions to the solutions, and other degradation routes induced at the rubber-liquid interface, as explain ahead.
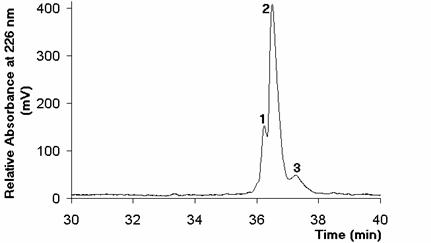
Figure 2. Effect of chlorobutyl stoppers on the RP-HPLC profile of rhIFNa-2b after 30 days of storage at 37 ºC. Peak 1: early eluting species; peak 2: rhIFN-a2b main peak; 3: moiety eluting just after the rhIFN-a2b main peak.
In order to kinetically evaluate the stability of the cytokine, we determined the percent of the area of the main native rhIFN-a2b peak as well as the area under this chromatographic peak. These two parameters offered an idea of the purity and the true quantity of unmodified rhIFN-a2b, respectively. The acceleration of the thermal inactivation resulted in a decline of both parameters with time. However, the decomposition effect was remarkably greater for the area under the chromatographic peak (area). This fact might be explained due to the probable occurrence of degradation routes that affect the area (e.g., precipitation and adsorption), without affecting the purity.
Influence of the concentration of rhIFN-a2b on the liquid stability of this cytokine in contact with chlorobutyl stoppers
Samples at the lowest concentration of the active ingredient (3 MIU/ml) that were stored without contact with stoppers reduced the loss of the purity and area in about 1.82- and 1.28-fold respectively, as compared to those samples that were stored in contact with chorobutyl stoppers (Fig. 3; Table 2). Similarly, 10 MIU/ml rhIFN-a2b stored without contact with gum stoppers decreased the loss of the same parameters in about 1.86- and 1.25-fold, respectively, compared to those solutions in contact with this packaging material (Table 2). Note that protein concentration affected this behavior. Consequently, 3 MIU/ml rhIFN-a2b decreased the RP-HPLC-determined parameters in a lower extension than 10 MIU/ml rhIFN-a2b solutions (Table 2). Kinetic analyses from the comparison between samples at both concentrations showed that diluted solutions that were stored without contact reduced the loss of the purity and area in about 1.43- and 1.22-fold respectively, compared to the concentrated samples at the same storage condition (Table 2).
Similar results were obtained when protein solutions were stored in contact with stoppers. In this case, kinetic analyses indicated that 3 MIU/ml rhIFN-a2b decreased the loss of the purity and area in 1.47- and 1.18-fold, respectively, compared to 10 MIU/ml rhIFN-a2b solutions (Table 2).
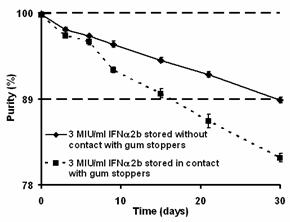
Figure 3. Effect of chlorobutyl stoppers on the RP-HPLC-determined purity of rhIFNa-2b. The cytokine rhIFNa-2b was used at 3 MIU/ml.
It has been suggested that the increase of the protein concentration to higher than 0.02 mg/ml may facilitate the potential aggregation or other degradation routes in these macromolecules (15). This influence can be explained due to the increment on the intermolecular collisions that usually increase the degradation of proteins (16).
The effect of the gum stoppers combined with the high temperature could increase the protein damage due to the adsorption-induced degradation processes (3). This process is usually concentration-dependent, therefore, we might expect a higher interface-induced degradation at higher concentrations of rhIFN-a2b.
Table 2. Kinetic parameters of the influence of chlorobutyl stoppers on the rhIFN-a2b thermal degradation at two concentrations of the cytokine.
| Concentrations |
In contact with chlorobutyl stoppers |
||||
| Puritya (k x 103 (day-1)) |
Areab (k x 103 (day-1)) |
||||
3 MIU/ml |
6.74 ± 1.22 |
39.11 ± 2.84 |
|||
| 10 MIU/ml |
9.92 ± 0.96 |
46.34 ± 3.11 |
|||
| Without contact with chlorobutyl stoppers |
|||||
| Puritya (k x 103 (day-1)) |
Areab (k x 103 (day-1)) |
||||
3 MIU/ml |
3.71 ± 0.62 |
30.47 ± 3.61 |
|||
10 MIU/ml |
5.32 ± 0.85 |
37.12 ± 3.14 |
|||
| aDetermination of the purity of the native rhIFN-a2b, as determined by RP-HPLC. bDetermination of the recovery of area under the peak corresponding to the native rhIFN-a2b, as determined by RP-HPLC. Results are expressed as mean (n=3) ± standard deviation (SD). |
|||||
Table 3. rhIFN-a2b thermal degradation in the presence of different buffer species.
| Buffer species |
In contact with chlorobutyl stoppers |
||||
| Puritya (k x 103 (day-1)) |
Areab (k x 103 (day-1)) |
||||
Sodium citrate |
5.52 ± 0.14 |
29.55 ± 2.41 |
|||
| Sodium citrate-phosphate |
6.15 ± 0.31 |
32.51 ± 3.62 |
|||
| Sodium phosphate |
6.74 ± 0.38 |
39.13 ± 2.95 |
|||
| Without contact with chlorobutyl stoppers |
|||||
| Puritya (k x 103 (day-1)) |
Areab (k x 103 (day-1)) |
||||
Sodium citrate |
2.75 ± 0.23 |
20.21 ± 2.13 |
|||
| Sodium citrate-phosphate |
3.21 ± 0.14 |
23.15 ± 2.46 |
|||
Sodium phosphate |
3.72 ± 0.29 |
33.45 ± 2.62 |
|||
| aDetermination of the purity of the native rhIFN-a2b, as determined by RP-HPLC. bDetermination of the recovery of area under the peak corresponding to the native rhIFN-a2b, as determined by RP-HPLC. Results are expressed as mean (n=3) ± standard deviation (SD). |
|||||
Influence of the buffer species on the liquid stability of rhIFN-a2b in contact with chlorobutyl stoppers
Buffer species affected the grade of the protein degradation (Table 3). Although the RP-HPLC profile was identical to that described before (Fig. 2), rhIFN-a2b prepared in sodium citrate and sodium citrate-phosphate and stored without contact with chlorobutyl stoppers reduced the loss of the purity in 1.35- and 1.16–fold, respectively, compared to those prepared in sodium phosphate buffer (Table 3). Similar kinetic results were obtained from the analysis of the area. In this case, inhibition of the loss of this parameter in samples prepared in sodium citrate and sodium citrate phosphate buffers was 1.65- and 1.2-fold greater, compared to those prepared in sodium phosphate buffer (Table 3).
Table 4. Kinetic parameters of the influence of chlorobutyl stoppers on the rhIFN-a2b thermal degradation in the presence of EDTA Na2 x 2H2O and polysorbate 80.
| Additives |
In contact with chlorobutyl stoppers |
||||
| Puritya (k x 103 (day-1)) |
Areab (k x 103 (day-1)) |
||||
EDTA Na2 x 2H2O |
5.01 ± 0.33 |
25.51 ± 3.57 |
|||
| Polysorbate 80 |
4.24 ± 0.24 |
13.37 ± 2.82 |
|||
| Without contact with chlorobutyl stoppers |
|||||
| Puritya (k x 103 (day-1)) |
Areab (k x 103 (day-1)) |
||||
EDTA Na2 x 2H2O |
2.39 ± 0.22 |
18.72 ± 2.71 |
|||
Polysorbate 80 |
2.01 ± 0.14 |
8.21 ± 3.25 |
|||
| aDetermination of the purity of the native rhIFN-a2b, as determined by RP-HPLC. bDetermination of the recovery of area under the peak corresponding to the native rhIFN-a2b, as determined by RP-HPLC. Results are expressed as mean (n=3) ± standard deviation (SD). |
|||||
These results indicated that the stability of rhIFN-a2b is considerably higher in sodium citrate and sodium citrate-phosphate buffers. Differences on the role of these buffer species on the stability of proteins have been previously discussed (17). Chen et al. found that citrates decreased the aggregation rate of the keratinocyte growth factor (17). They speculated that this effect was explained due to the presence of negative charges in the buffer ions, which interact with the positive amino acid clusters on the protein. Thus, the protein was stabilized through this charge-charge interaction (17).
Taking into account this speculation we should expect a similar stabilization of other proteins like rhIFN-a2b in the presence of the sodium phosphate buffer due to the negative charges that phosphate ions exhibit. However, our results were largely different (Table 3). The general explanation could be based on the presence of the trace amounts of metal ions on these salts which may accelerate the protein damage (18). In contrast, sodium citrate and sodium citrate-phosphate buffers stabilized rhIFN-a2b on the basis of the combination of the abovementioned mechanism (charge-charge interaction) together with the ability of citrates to act as chelating agents of the trace amounts of metal ions that could be present in protein solutions. In general, the contact with chlorobutyl stoppers accelerated the protein degradation in all the three evaluated buffers (Table 3).
The impact of the buffer composition on the protection against the stopper-induced degradation was estimated through the comparison of the results that were obtained at both, with and without contact with chlorobutyl stoppers. As a result, sodium citrate-, sodium citrate-phosphate- and sodium phosphate-based samples that were stored in contact with gum stoppers, reduced the purity of rhIFN-a2b in about 2-, 1.91- and 1.81-fold respectively, compared to the same samples stored without contact with this primary pack (Table 3). Similarly, analyses of the area showed a reduction on this parameter in 1.46-, 1.4- and 1.17-fold, respectively, for the same solutions stored in contact with chlorobutyl stoppers, compared to the same samples stored without contact with this packaging material (Table 3). It seems that buffers containing citrates had a higher impact on the inhibition of the stoppers-induced degradation of this protein.
Given the ineffectiveness of citrates to completely inhibit the stoppers-induced degradation of rhIFN-a2b, we can speculate that the release of heavy metal ions from the chlorobutyl gum to aqueous solution is not the main mechanism involved on the deleterious effect of this protein when it is stored in contact with gum stoppers. It seems that other factors like the presence of metal ions in the salts of the buffers may increase the degradation of rhIFN-a2b at both conditions, with and without contact with chlorobutyl stoppers. Therefore, other mechanisms (e.g. adsorption-induced degradation at liquid/stoppers interfaces) might explain the influence of the gum stoppers on the stability of rhIFN-a2b.
Influence of polysorbate 80 and EDTA Na2 x 2H2O on the liquid stability of rhIFN-a2b in contact with chlorobutyl stoppers
In order to elucidate the main mechanism that explains the effect of the gum stoppers on the stability of rhIFN-a2b, we evaluated the influence of two additives. One of such additives, EDTA Na2 x 2H2O, is a chelating agent that has been frequently used to stabilize proteins because of its ability to bind any harmful metal ion (3). The second one, polysorbate 80, is a nonionic surfactant that drops the surface tension for protein solutions and decreases the forces that drive proteins to aggregation by hydrophobic interactions (6).
In this experiment, EDTA Na2 x 2H2O increased the purity and the area of rhIFN-a2b in samples that were stored without contact with gum stoppers in about 2.09- and 1.36-fold compared to the samples stored in contact with this packaging material (Table 4). This fact might indicate that one possible degradation mechanism could be the delivery of metal ions from the gum stoppers to the solution. Nevertheless, we discarded this possibility given the ineffectiveness of EDTA Na2 x 2H2O to completely eliminate the stopper-induced degradation of rhIFN-a2b. However, the presence of contaminating metal ions in the buffer salts (as described before) which can accelerate the damage of the protein, and its binding by EDTA Na2 x 2H2O, might also explain the effect of this chelator on the preservation of the stability of rhIFN-a2b.
On the other hand, results from experiments with polysorbate 80 indicated that this detergent increased the purity and the area of the cytokine in samples stored without contact with chlorobutyl stoppers in about 2.11- and 1.63-fold, respectively, compared to those stored in contact with this primary pack (Table 4). From these data, it is evident that the non ionic detergent was not absolutely effective on the preservation against the stopper-induced degradation. However, it might be explained due to the probable presence of metal ions in the buffer salts that could accelerate the thermal degradation of rhIFN-a2b. In any case, polysorbate 80 was more effective than EDTA Na2 x 2H2O against the stopper-induced degradation of this protein, specially, in the analysis of the area of the main rhIFN-a2b peak, as kinetic evaluation indicated (Table 4). These evidences pointed to that the protein damage induced at the stopper/liquid interfaces could be the most important mechanism explaining the deleterious effect of chlorobutyl stoppers on the stability of rhIFN-a2b.
Conclusions
The compatibility of rhIFN-a2b in sodium phosphate buffer with type I borosilicate glass ampoules showed a significant adsorption at the lowest concentration of the active ingredient. However this effect was absolutely eliminated when a polysorbate 80/benzyl alcohol-based vehicle was used instead of the sodium phosphate buffer. On the other hand, the heat sealing of ampoules affected the stability of rhIFN-a2b when a 1-ml volume was dispensed to these vials. In contrast, this effect was not found with a lower (0.5 ml) volume. Additionally, experimental data indicated that the contact with stoppers might accelerate the degradation of rhIFN-a2b, probably due to adsorption-induced destabilization mechanisms. However, this effect can be modulated as a function of the concentration of the protein and the use of different buffer species and excipients.
References
1. Zoon, K., zur Nedden, D., Enterline, J., Manischewitz, J., Dyer, D., Boykins, R., Gerrard, T., Chemical and biological characterization of natural human lymphoblastoid interferon alphas. Biology of the interferon system. Eds., Martenus Nijkoff, Publishers, Dertecht, 567, 1986.
2. Bordens, R., Grossberg, S. E., Trotta, P. P. and Nagabhushan, T. L., Molecular and biologic characterization of recombinant interferon-alpha2b. Semin. Oncol., 24 (3 Suppl 9): S9-41-S9-51, 1997.
3. Wang, W., Instability, stabilization, and formulation of liquid protein pharmaceuticals. Int. J. Pharm., 185 (2): 129-188, 1999.
4. Pikal, M. J. and Shah, S., Moisture transfer from stopper to product and resulting stability implications. Dev. Biol. Stand., 74: 165-77, 1992.
5. Chang, B. S., Kendrick, B. S. and Carpenter, J. F., Surface-induced denaturation of proteins during freezing and its inhibition by surfactants. J. Pharm. Sci., 85(12): 1325-30, 1996.
6. Bam, N. B., Cleland, J. L., Yang, J., Manning, M. C., Carpenter, J. F., Kelley, R. F. and Randolph, T. W., Polysorbate protects recombinant human growth hormone against agitation-induced damage via hydrophobic interactions. J. Pharm. Sci., 87(12): 1554-1559, 1998.
7. Earle, J. P., Bennett, P. S., Larson, K. A. and Shaw, R., The effects of stopper drying on moisture levels of Haemophilus influenzae conjugate vaccine. Dev. Biol. Stand., 74: 203-10, 1992.
8. Padrón, G., Besada, V., Agraz, A., Quiñonez, Y., Herrera, L. and Shimonishi, Y., Spectrometric analysis of recombinant human a-2 Interferon. Analytica Chimica Acta, 223: 361-9, 1989.
9. Beldarraín, A., Cruz, C., Cruz, O., Navarro, M. and Gil., M., Purification and conformational properties of a human interferon alpha 2b produced in Escherichia coli. Biotechnol. Appl. Biochem., 33: 173-182, 2001.
10. Cruz, S., Duarte, C., Ferra, E., Fonterroche, G., Vázquez, J., Martínez, L., Arteaga, N., Pérez, E. and Gavilondo, J., Cuantificación de interferón alfa 2b humano recombinante mediante anticuerpos monoclonales. Biotecnología Aplicada, 7: 132-141, 1990.
11. Santana, H., Espino, Y., Franco, A., Furrazola, G. and Hardy, E., A sandwich-type enzyme-linked immunosorbent assay for the analysis of recombinant human interferon a-2b. Biotech. Tech., 5: 341-346, 1999.
12.
Sigarroa, A., Biometry and Experimental Design,
13.
Gross, G., Del Terzo, S. and Kumar, S., Stabilizer interferon alpha
solutions.
14.
Yuen, P. and Kline, D., Stable aqueous alpha interferon solution formulations.
15. Ruddon, R. W. and Bedows, E., Assisted protein folding. J. Biol. Chem., 272: 3125, 1997.
16. Rupley, J. A. and Careri, G., Protein hydration and function. Advances in Protein Chemistry, 41: 37-173, 1991.
17. Chen, B. L., Arakawa, T., Morris, C. F., Kenney, W. C., Wells, C. M. and Pitt, C. G., Aggregation pathway of recombinant human keratinocyte growth factor and its stabilization. Pharm. Res., 11(11):1581-7, 1994.
18. Fransson, J. and Hagman, A., Oxidation of human insulin-like growth factor I in formulation studies, II. Effects of oxygen, visible light, and phosphate on methionine oxidation in aqueous solution and evaluation of possible mechanisms. Pharm. Res., 13(10): 1476-81, 1996.
Corresponding author: Llamil Ruiz, Formulation Development Department, Center
for Genetic, Engineering and Biotechnology (CIGB),
Email: llamil.ruiz@cigb.edu.cu
Published by the Canadian Society for Pharmaceutical Sciences.
Copyright © 1998 by the Canadian
Society for Pharmaceutical Sciences.
http://www.cspscanada.org
CSPS Home | JPPS
Home | Search
| Subscribe to JPPS