J Pharm Pharmaceut Sci (www.cspscanada.org) 8(2):132-140, 2005
Preparation of sustained release hydrophilic matrices by melt granulation in a high-shear mixer.
Lourdes Ochoa, Manuela Igartua, Rosa Ma, Hernández, Alicia R. Gascón, José Luis Pedraz1
Laboratory of Pharmacy and Pharmaceutical Technology, Faculty of Pharmacy, University of the Basque Country (UPV-EHU), Paseo de la Universidad nº7, Vitoria-Gasteiz, Spain12 November 2004, Revised 21 February 2005, Accepted 28 February 2005, Published 29 June 2005
Abstract
Purpose: The objective of this work was to prepare theophylline sustained release matrix tablets based on the combination of hydroxypropyl methylcellulose (HPMC K4M and K100M) and different meltable binders by melt granulation in a high-shear mixer. Methods: Dissolution profiles of each formulation were compared to those of TheoDur® 200 mg tablets and the mean dissolution time (MDT) and similarity factor (f2 factor) were calculated. The matrices swelling behavior was investigated by texture analysis. Results: The results obtained show that the type of excipient influenced the drug release rate. In particular, the dissolution rate was delayed when lipophilic binders were used and only formulations containing Gelucire® 50/13 or PEG 6000 with HPMC K4M had a profile similar to the commercial formulation. The release mechanism of theophylline from the formulations was described by Peppas's equation showing a non-Fickian release mechanism. The investigation of matrices swelling behavior showed that the gel layer thickness increased continuously over the time period studied. Moreover, a correlation between gel layer thickness and strength with the percentage released was found. Conclusions: These results suggest that melt granulation could be an easy and fast method to formulate sustained release tablets.
Introduction
Development of sustained release oral dosage forms is beneficial for optimal therapy regarding efficacy, safety and patient compliance (1). Frequently used approaches to achieve adequate control of drug release include hydrophilic and lipophilic matrix systems, in which the mechanism of drug release is based on a combination of diffusion and erosion processes (2, 3). Among the numerous hydrophilic carrier materials tested for the development of hydrophilic matrices, the most commonly used is hydroxypropylmethylcellulose (HPMC), which has been used since the early 1960s (4, 5). Their properties as gelling agents are very important in the formulation because they are responsible for the formation, by hydration, of a diffusion and erosion-resistant gel layer which is able to control drug release (6). Studies reported from this laboratory in recent years have demonstrated the possibility of obtaining sustained release formulations of different drugs such as salbutamol and ketoprofen from hydrophilic matrices prepared with HPMC (7).
On the other hand, lipophilic materials have been also employed as matrix carriers for sustained release solid dosage forms (8, 9). In addition to direct compression and wet granulation, wax matrices can be prepared by solid dispersion (10) and melt granulation methods, suitable for granulation of hygroscopic drugs and hydrophilic materials such as HPMC. Melt granulation in a high-shear mixer (MG) is a single-step technique that converts fine powders into granules combining several processing steps into a single operation unit (11). The powder agglomeration is promoted by the addition of a low melting point binder, which is solid at room temperature and melts at relatively low temperatures (50-80ºC). The binder is liquefied by the heat generated either by a heating jacket or by the friction of the impeller blades during the mixing phase (12). This technique is a good alternative to the conventional wet granulation process when the use of solvents is not indicated. Moreover, it offers several advantages since the drying phase is eliminated and the process is less consuming in terms of time and energy (13). Besides, this procedure brings the possibility of preparing sustained release formulations by selecting the suitable binder and excipients (14).
Based on these observations, in the present study hydrophilic matrix systems were designed by compression of granules obtained by a high-shear melt granulation process. The purpose of this work was to develop sustained release tablets using theophylline, as a model drug, and HPMC in combination with different binder agents as sustained release excipients to tailor drug release to that of the commercial formulation. Due to the important role of swelling on drug release from water-swellable matrix systems (15, 16), texture analysis has been applied to investigate the swelling behavior of tablets during the hydration process.
Materials and Methods
Materials
Anhydrous theophylline obtained from Vencaser, S.A. (Bilbao, Spain) was used as model drug. HPMC (Methocel® K4M Premium and Methocel® K100M Premium, kindly supplied by Colorcon (Kent, U.K.), were used as starting materials and magnesium stearate (Kirsch Pharma, Madrid, Spain) as a lubricant. Gelucire® 50/13, Compritol® 888 Ato, Precirol® Ato 5 (Gattefossé, Barcelona, Spain), PEG 6000, Stearic acid and Glycerol monostearate (Vencaser, S.A., Bilbao, Spain) were used as meltable binders.
Granulation
The granules were prepared in a laboratory scale "one-step" high-shear mixer Rotolab® (Zanchetta, Italy) equipped with an electrically heated jacket using different lipophilic and hydrophilic binders.
The granulation procedure was standardized on the basis of preliminary trials. The mixture composed of theophylline (50 %), HPMC K4M or K100M (30 %) and a binder agent (20 %) were mixed at an impeller speed of 250 rpm for 5-10 min. Successively the mixture was heated with the heating jacket up to the melting point of the binder and the impeller speed was increased in order to obtain granules. At the end of the granulation process the granules were cooled at room temperature by decreasing the jacket temperature to 25ºC and tilting the bowl.
Granule characterization
a) Differential Scanning Calorimetry (DSC)
The DSC analyses were performed using a DSC-50 calorimeter/TAC-50 (Shimadzu Corp., Kyoto, Japan). Samples of about 5 mg were sealed in a 30 ml aluminium pan and were scanned between 10ºC and 300ºC at a heating rate of 10ºC/min.
b) Particle size
The granules obtained were sieved in order to remove lumps larger than 1 mm. The particle size distribution was determined using a Sympatec Helos/Rodos laser diffraction particle size analyzer (Sympatec GmbH., Clausthal-Zellerfeld, Germany) with dry powder dispersion capability and a vibrating conveyor feeder. The powder dispersion pressure was varied between 1.0 and 2.0 bars with direct feed into the dispersion funnel. Measurements were carried out in triplicate (n=3).
c) Flow properties
The flow properties of the granules can be evaluated by repose angle (a), Carr Index (CI) and Hausner ratio (HR). Repose angle was measured pouring the granules through a glass funnel onto a flat surface. The Carr Index and Hausner ratio were obtained by using a SBS Instruments tap density apparatus with a 250 ml glass-measuring cylinder.
Manufacture of tablets
The granules were mixed with 0.5 % magnesium stearate for 10 min using a rotating V-blender (Emjuvi, Barcelona, Spain) and compressed into tablets on a reciprocating single punch tablet machine (BONALS, Barcelona, Spain) at a breaking strength of 70-80 N. The breaking strength was determined using a Pharma-test GmbH PTB-311 model (Hainburg-Germany) durometer.
Tablets weighted around 400 mg ± 5% and contained 200 mg of theophylline. The tablet shape, size and hardness were held constant for all formulations.
In vitro dissolution studies
Drug release was performed using the USP 25 type II apparatus (Sotax AT7 dissolution tester, Allschwil, Switzerland) at a rotation speed of 50 rpm in 1000 ml distilled water at 37 ± 0.5ºC. Samples were extracted at regular time intervals during 24 h. The amount of drug released was assayed spectrophotometrically at 272 nm.
Dissolution kinetic
In order to characterize the mechanism of drug release from the matrices, the data were fitted to the following mathematical model (17, 18):
Eq. 1
where Mt/M¥ is the fraction of drug released up to time t, K is the kinetic constant and n is the release exponent indicative of the release mechanism.
The mean dissolution time (MDT) was also calculated. MDT is the mean ratio of the first to zero moments of the dissolution rate-time curve and is expressed by the following equation:
Eq. 2
where ACC is the complementary area under the accumulated dissolution curve and M¥ is the maximum accumulated dissolution amount.
Experimental data were fitted to Peppas's equations by using the WINNONLIN® program (19), and MDT calculations were performed with the same program.
The similarity factor (f2) is used to compare the dissolution profile of each formulation with that of marketed formulation. In this approach, recommended by the FDA Guidance for Industry, a value between 50 and 100 indicates similarity between two dissolution profiles. If the f2 value is close to 100, the two profiles are nearly identical (20, 21).
Gel texture analysis
In order to avoid deformation during the determination of core/gel interface, one planar base of the tablets was covered with an organic water insoluble coating (14 mg Eudragit® RS PO in a 1:1 mixture of isopropanol and acetone) and glued to a microscope slide. The samples were placed in dissolution vessels filled with 1000 ml distilled water at 37 ± 0.5ºC in the USP paddle apparatus at 50 rpm to simulate the in vitro dissolution process. The swollen tablets were taken out at predetermined intervals over a period of 12 h for texture analysis. Expansion of tablets during swelling process was investigated in triplicate using the Texture Analyzer (TA.XT2, Stable Micro System, Goldalming, UK), which provided information not only on gel layer thickness but also on the texture (strength) of the gel, as complete force-distance curves are recorded during the penetration process.
The penetration of a flat-tipped round steel probe (2 mm diameter and 30 mm height) into swollen matrices was determined at a test speed of 0.1 mm/s under increasing load, and data collection and analyses were performed by a computer equipped with the Texture Expert® software. A predetermined maximum force of 800 g was established in order to differentiate the glassy polymer from gelled polymer.
Results and Discussion
Differential Scanning Calorimetry studies
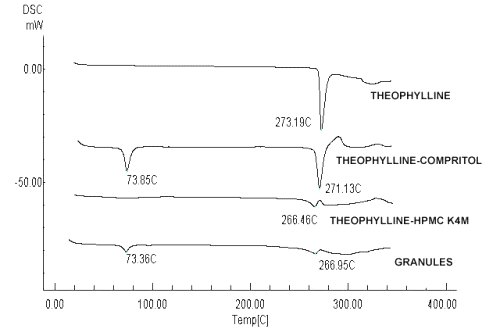
The DSC curves were obtained for theophylline, excipients, their physical mixture with the same proportion of the tablets, and granules obtained by melt granulation. The DSC analysis of theophylline showed a single endothermic peak at 273.20 ± 2ºC, due to the melting of the drug. HPMC K4M and K100M did not show any characteristic peak, and binder agents showed their respective peak at their melting range (Compritol®: 69-74ºC, Glycerol monostearate: 55-60ºC, Stearic acid: 55-60ºC, Gelucire® 50/13: 46-50ºC, Precirol®: 53-57ºC and PEG 6000: 55-63ºC). In the DSC curves of physical mixtures and melt granules the characteristic peaks of theophylline and binder agents were almost unchanged indicating the absence of strong interactions between the components and suggesting drug-excipient compatibility in all the formulations examined.
Figure 1 shows an example of the thermograms obtained for theophylline, its physical mixture with Compritol® or HPMC K4M and the corresponding granules.
Figure 1: DSC thermograms obtained for theophylline, the physical mixture with Compritol® or HPMC K4M and the corresponding granules.
Particle size and flow properties
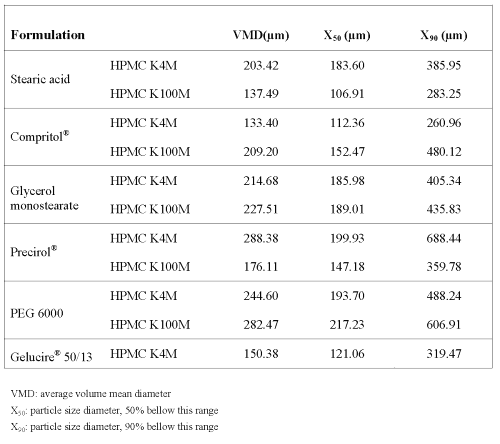
As shown in table 1, particle size distribution measurements showed that 90% of the granules elaborated had diameters <690 mm, and the mean particle size values of the granules ranged between 130-290 mm for all the batches prepared. The particle size distribution was unimodal showing that the different excipients used did not influence the granules particle size. Taking flow properties into account, satisfactory values for Hausner Ratio (HR), Carr Index (CI) and repose angle (a) were obtained for all formulations. The HR values obtained were below 1.25 and repose angle was around 34º, indicating that the granules showed good flowability (22). Moreover, the values of CI were below 15%, which means that the granules have good flow properties, thus suggesting the possibility of continuous tabletting (23). However, it was not possible to compress the granules prepared with Gelucire® 50/13 and HPMC K100M.
Table 1: Size distribution of granules.
Drug release profiles
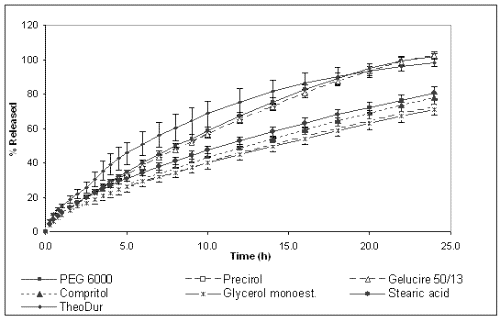
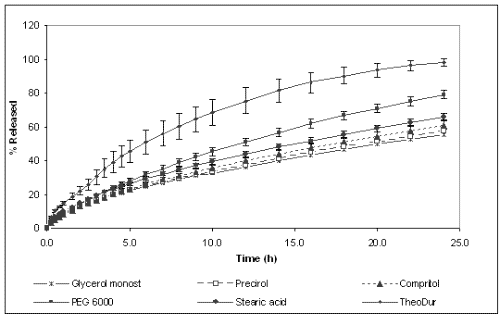
Figures 2 and 3 show the dissolution profiles of theophylline for the formulations prepared with different binders and HPMC K4M or K100M, respectively, and TheoDur® 200 mg.
Figure 2: Cumulative percentage of theophylline released from different formulations prepared with HPMC K4M.
Figure 3: Cumulative percentage of theophylline released from different formulations prepared with HPMC K100M.
The marketed formulation was found to provide a sustained release for a period of 24 h. In vitro dissolution results show that the matrices prepared with granules obtained by melt granulation provided an extended release of the drug. However, different release behavior was observed depending on the binder used for their preparation. On one hand, the formulations prepared with lipophilic binders (Compritol® 888 Ato, Precirol® Ato 5, Stearic acid and Glycerol monostearate) showed the slowest release rate and their dissolution profiles were almost superimposable. After 24 h only around 60-80 % of the total drug amount was released for both HPMC K4M and K100M celluloses. The lipophilic binders could probably make the wetting of the solid matrix difficult and, subsequently, allow a slower release rate. On the other hand, the formulations containing PEG 6000 and Gelucire® 50/13 and HPMC K4M achieved the total release of the drug at 24 h, whereas the formulation with PEG 6000 and HPMC K100M only released 80%. These differences between cellulose grades can be explained by the fact that the gel layer of HPMC K100M was more viscous and less erodible than that of HPMC K4M, providing a stronger barrier for drug diffusion, and resulting in a slower drug release rate (24).
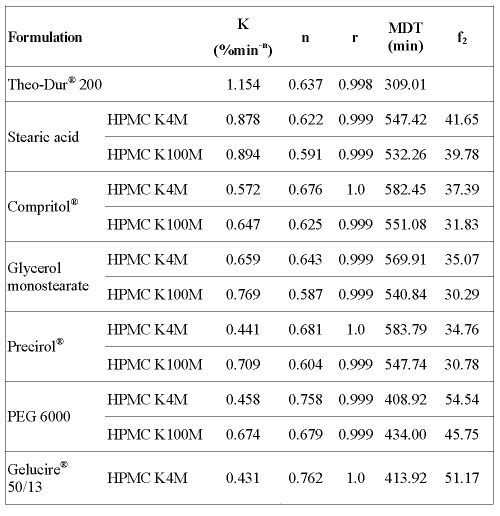
Release data from swellable HPMC systems can be analyzed according to Peppas's equation. The values of the kinetic parameters obtained from the data fitting to the power law equation (Eq. 1) and MDT (Eq. 2) are listed in table 2.
Table 2: Values of the kinetic parameters and MDT.
MDT values obtained for the matrices prepared with HPMC K4M show that formulations containing a lipophilic binder such as Stearic acid, Compritol®, Glycerol monostearate and Precirol® (MDT = 547.42; 582.45; 569.91 and 583.79 min, respectively), released the drug at a slower rate and to a lesser extent than those containing an hydrophilic one (Gelucire® 50/13 and PEG 6000) or the commercial formulation (MDT = 413.92; 408.92 and 309.01 min, respectively). Similar results were observed for HPMC K100M formulations where PEG 6000 (MDT = 434.00) showed a faster drug release than lipophilic binders (table 2). Furthermore, the value of exponent n can be used to characterize the release mechanism of controlled release matrices. According to the values shown in table 2, between 0.5 and 1, one may conclude that the drug release during the dissolution test is controlled by both diffusion of the drug through the hydrated matrix and the erosion of the matrix itself; that is, a non-Fickian (anomalous) solute diffusion mechanism (17, 18).
The similarity factor (f2) was also calculated in order to compare each formulation profile with that of the reference formulation. The values of f2 are listed in table 2. Comparison of the profiles indicated that only the formulations containing HPMC K4M and Gelucire® 50/13 or PEG 6000 had a profile similar to the commercial formulation (f2 = 51.17 and 54.54, respectively). However, the other formulations did not provide the desired sustained release profile (f2 < 50).
Gel texture analysis
According to the investigations of Yang et al. (16) the Texture Analyzer was used in an effort to correlate the gel layer thickness with the dissolution profiles of matrix tablets. This test measures the force necessary for penetration of the probe into the swollen tablet.
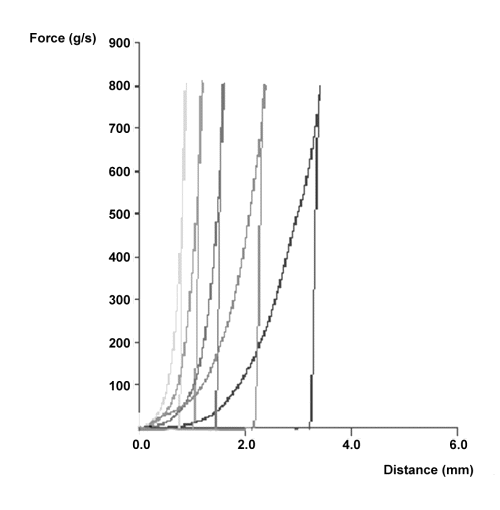
Figure 4 illustrates the penetration profiles obtained for the Compritol®-HPMC K4M tablets at different swelling times. As can be seen in the figure, at the beginning of the measure only small forces are necessary to penetrate the swollen gel layer. Then, a progressive increase is observed up to a sharp raise in the force required for further penetration indicating the position of the boundary between gel layer and the dry core. Once the maximum force is reached, reverse movement starts withdrawing the probe from the swollen tablet. Gel layer thickness was obtained calculating the maximum penetration distance of the probe into the swollen tablets.
Figure 4: Force-distance profiles of Compritol®-HPMC K4M tablets at different swelling times (from left to right: 1, 2, 4, 8 and 12 hours).
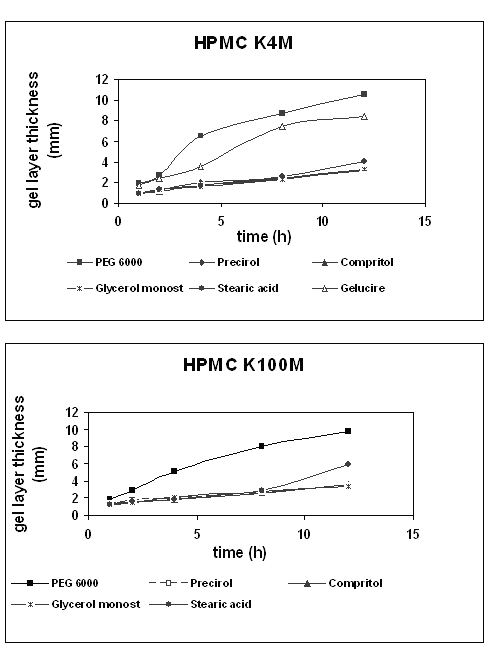
Figure 5 summarizes the results obtained for the hydration process of theophylline matrices based on HPMC K4M or K100M as a function of time of exposure to an aqueous environment.
Figure 5: Swelling behavior of HPMC K4M and K100M tablets as a function of time of exposure to an aqueous environment.
As might have been expected, a different swelling behaviour was found for hydrophilic and lipophilic binders, similar for both HPMC grades. The penetration profiles of the tablets prepared with hydrophilic binders showed a strong increase in the penetration distance with swelling time, indicating a significant grow in gel layer thickness. In contrast, the formulations containing lipophilic binders showed a continuous but less remarkable increase with time in gel layer thickness. Comparing the swelling profiles obtained, significant differences between hydrophilic and lipophilic formulations are observed. After a -one- hour swelling, the penetration distance of the probe was similar in all cases (0.9-1.2 mm). Nevertheless, after a 12 h swelling the distance penetrated for hydrophilic binder formulations was higher (8-10 mm for PEG 6000 and Gelucire® 50/13) than for the lipophilic ones (about 3-5 mm). More remarkable was the case of Stearic acid and HPMC K100M, which showed a sharp increase of the gel layer thickness at the last point. These results support those obtained in the dissolution studies. On the one hand, tablets with hydrophilic binders were more hydrated, and fast polymer hydration caused greater release of the drug. On the other, all lipophilic binders had a similar and less pronounced swelling and their dissolution profiles were also identical, leading to a slower and to a lesser extent drug release than those containing hydrophilic binders.
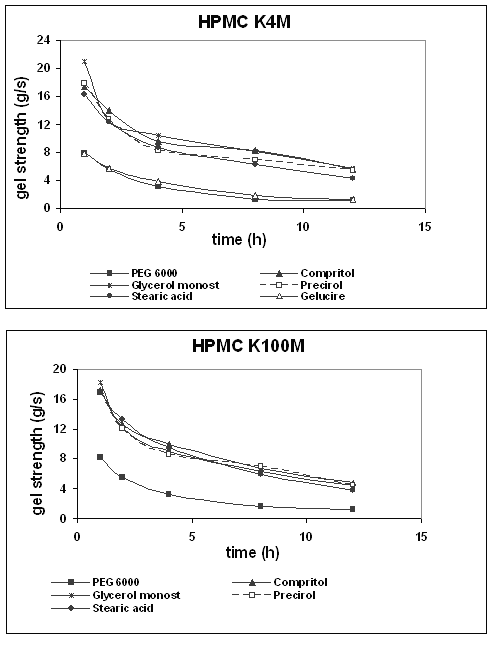
Usually, during the swelling process, a gradient in gel layer hydration is developed. Outer areas are characterized by lower gel strength (low polymer concentration) and less resistance to penetration, while less hydrated areas of more dense gel structure require higher force for penetration. Comparing long and short swelling times, it becomes obvious that prolonged swelling time did not only lead to an increase in the maximum penetration distance but also altered the load needed for penetration significantly (25). In order to support this idea, the slope of the texture analysis curves was measured between zero and 100 grams. Figure 6 illustrates the values obtained for different tablet formulations when varying the swelling time.
Figure 6: Gel strength (slope of the texture analysis curves) of HPMC K4M and K100M tablets as a function of time of exposure to an aqueous environment.
Since a behaviour similar to that of penetration distance was observed for hydrophilic and lipophilic binders, respectively, the formulation with the highest gel layer thickness (PEG 6000 and HPMC K4M) and the lowest one (Glycerol monostearate and HPMC K100M) were compared. In the case of PEG 6000, after swelling for 1 h, the slope of the curve was about 8 g/s and the penetration distance was almost 2 mm. At this point, Glycerol monostearate showed an 18.2 g/s slope approximately and the probe advanced 1.2 mm.
After 12 h swelling, the slopes were 1.1 and 4.5 g/s and the maximum penetration distances were 10.5 and 3.5 mm for PEG 6000 and Glycerol monostearate, respectively. These differences can be attributed to the lower hydration and bigger gel strength of the HPMC with lipophilic binder.
Finally, the influence of matrix swelling, gel layer thickness and the slope (gel strength) of the force-distance curve on the release of theophylline was evaluated by multiple regression analysis using "Minitab 14.10" software (26). The percentage of theophylline released was found to be dependent on these parameters according to the following equations obtained by multiple regression analysis:
Hidrophilic binder formulations:
Eq. 3
Lipophilic binder formulations:
Eq. 4
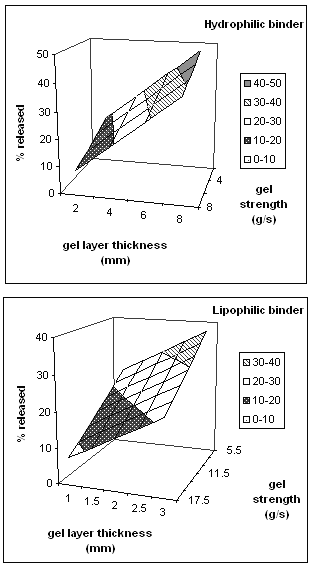
Figure 7 shows the calculated response surfaces for theophylline release from HPMC matrices as a function of gel layer thickness and texture analysis curve slope (gel strength).
Figure 7: Response surface plots for theophylline release from HPMC matrices as a function of gel layer thickness and texture analysis curve slope (gel strength).
As might have been expected, the percentage released correlated with the extent of hydration, an increase of gel thickness and a decrease in the curve slope led to an increase in the amount of drug released.
Conclusions
The present study has shown that "one-step" melt granulation in high-shear mixer could be a viable method to prepare sustained release formulations.
The type of excipient influenced the rate and extent of drug release from melt granulation based matrix tablets. Comparison of dissolution profiles indicated that only the formulations containing HPMC K4M and Gelucire® 50/13 or PEG 6000 had a profile similar to the commercial formulation studied. However, the release of theophylline was remarkably lowered when liphophilic binders were used.
Additionally, the swelling study with the Texture Analyzer allows us to correlate the dissolution profiles with the thickness and strength of the gel layer formed during the hydration process.
Acknowledgements
We address our thanks to Dr. Salmerón for his technical assistance with the texture analyzer.
References
Y.W.Chien, Controlled and modulated-release drug delivery systems, in: J. Swarbrick and J.C. Boyland (Ed.), Encyclopedia of Pharmaceutical Technology, Marcel Dekker, Inc., New York, 1990, pp. 281-313.
T. Gren, C. Nyström, Porous cellulose matrices containing lipophilic release modifiers- a potential oral extended-release system, Int. J. Pharm. 184 (1999) 7-19.
S. Kiil, K. Dam-Johansen, Controlled drug delivery from swellable hydroxypropylmethylcellulose matrices: model-based analysis of observed radial front movements, J. Control. Rel. 90 (2003) 1-21.
A. T. Pham, P.I. Lee, Probing the mechanism of drug release from hydroxypropylmethyl cellulose matrices, Pharm. Res. 11(10) (1960) 1379-1384.
R.M. Hernández, A.R. Gascón, B. Calvo, C. Caramella, U. Conte, A. Domínguez-Gil, J.L. Pedraz, Correlation of “in vitro” release and “in vivo” absortion characteristics of four salbutamol sulfate formulations, Int. J. Pharm. 139 (1996) 45-52.
M. J.Vazquez, B. Pérez-Marcos, J.L. Gómez-Amora, R. Martínez-Pacheco, C. Souto, A. Concheiro, Influence of technological variables on release of drugs from hydrophilic matrices, Drug. Dev. Ind. Pharm. 18 (1992) 1355-1375.
M.A. Solinís, Y. De La Cruz, B. Calvo, R.M. Hernández, A.R. Gascón, I. Goñi, M.D. Gurruchaga, J.L. Pedraz, Release of Salbutamol sulfate and Ketoprofen enantiomers from matrices containing HPMC and cellulose derivates, Chirality 14 (2002) 806-813.
R. Thies, P. Kleinebudde, Melt pelletisation of a hygroscopic drug in a high-shear mixer. Part 1. Influence of process variables, Int. J. Pharm. 188 (1999) 131-143.
D. Voinovich, M. Moneghini, B. Perissutti, J. Filipovic-Gecic, I. Grabnar, Preparation in high-shear mixer of sustained-release pellets by melt pelletisation, Int. J. Pharm. 203 (2000) 235-244.
A. Esquisabel, A. San Vicente, M. Igartua, R.M. Hernández, A.R. Gascón, B. Calvo, J.L.Pedraz, Influence of melting point and hydrophilic/lipophilic balance on the release of salbutamol sulfate from lipid matrices, S.T.P. Pharma. Sci. 6(5) (1996) 365-369.
A. Royce, J. Suryawanshi, U. Shah, K. Vishnupad, Alternative Granulation Technique: Melt granulation, Drug. Dev. Ind. Pharm. 22 (1996) 917-924.
C.M. Mc Taggart, J.A. Ganley, A. Sickmneller, S.E. Walder, The evaluation of formulation and processing conditions of a melt granulation process, Int. J. Pharm. 19 (1984) 139-148.
T. Schaefer, P. Holm, H.G. Kristensen, Melt granulation in a laboratory scale high-shear mixer, Drug. Dev. Ind. Pharm. 16 (1990) 1249-1277.
K.J. Crowley, R.T. Forbes, P. York, H. Nyqvist, O. Camber, Drug-fatty acid salt with wax-like properties employed as binder in melt granulation, Int. J. Pharm. 211 (2000) 9-17.
R. Konrad, A. Christ, G. Zessin, U. Cobert, The use of ultrasound and penetrometer to characterize the advancement of swelling and eroding fronts in HPMC matrices, Int. J. Pharm. 163 (1998) 123-131.
L. Yang, B. Johnson, R. Fassihi, Determination of continuous changes in the gel layer thickness of poly(ethilene oxide) and HPMC tablets undergoing hydration: a texture analysis study, Pharm. Res. 15 (1998) 1902-1906.
N.A. Peppas, Analysis of Fickian and Non-Fickian drug release from polymers, Pharm. Acta. Helv. 60 (1985) 110-111.
R.W. Korsmeyer, R. Gurny, E. Doelker, P. Buri, N.A. Peppas, Mechanism of solute release from porous hydrophilic polymers, Int. J. Pharm. 15 (1983) 25-35.
WINNOLIN®. 1995. Scientific Consulting Inc. North Carolina USA..
V.P. Shah, Y. Tsong, P. Sathe, J. Liu, In vitro dissolution profile comparison-statistics and analysis of the similarity factor, f2, Pharm. Res. 15 (1998) 889-896.
J.W. Moore, H.H. Flanner, Mathematical comparison of curves with an emphasis on in vitro dissolutions profiles, Pharm. Technol. 20 (1996) 64-74.
H.H. Hausner, Effect of quench sintering on the grain structure of sintered metals, Int. J. Powder. Metall. 8(3) (1972) 159-161.
R.L.Carr, Evaluating flow properties of solids, Chem. Eng. 72(2) (1965) 163-168.
J. Liu, F. Zhang, J.W. McGinity, Properties of lipophilic matrix tablets containing phenylpropanolamine hydrochloride prepared by hot-melt extrusion, Eur. J. Pharm. Biopharm. 52 (2001) 181-190.
- S. Zuleger, R. Fassihi, B.C. Lippold, Polymer particle erosion controlling drug release. II. Swelling investigations to clarify the release mechanism, Int. J. Pharm. 247 (2
Minitab® Release 14 Statistical Software. 2003. Minitab Inc. PA USA.
Corresponding Author: Prof. J.L. Pedraz Muñoz, Laboratory of Pharmacy and Pharmaceutical Technology, Faculty of Pharmacy, University of the Basque Country (UPV-EHU), Paseo de la Universidad nº 7, 01006 Vitoria-Gasteiz. Spain. knppemuj@vf.ehu.es
Published by the Canadian Society for Pharmaceutical Sciences.
Copyright © 1998 by the Canadian Society for Pharmaceutical Sciences.
http://www.cspscanada.org