J Pharm Pharmaceut Sci (www.cspscanada.org) 8(2):217-225, 2005
Pharmacists and Prescribing Rights: Review of International Developments
Lynne Emmerton,1 Jennifer Marriott,2 Tracey Bessell,2 Lisa Nissen,1 Laura Dean2
Received December 12, 2004, Revised May 19, 2005, Accepted May 20, 2005, Published, August 4, 2005
ABSTRACT. Purpose. Continuity of care, equitable access, and quality and safety are major foci in health services management. The introduction of limited prescribing rights to pharmacists has the potential to reduce fragmentation within the health system, optimise medication management, improve continuity of patient care and improve patient access to medication. Results. Eight models for pharmacists’ prescribing have been implemented internationally, varying in their dependency on protocols, formularies and collaboration with physicians. These have also been described using terms such as Supplementary Prescribing and Patient Group Directions. Conclusion. Issues relating to practical implementation of pharmacists’ prescribing include negotiation of national health policy, pharmacists’ training and accreditation, liability, reimbursement and documentation.
INTRODUCTION
There has been significant change in the supply of medicines over the last few decades. Technology has made recording of prescriptions less time-consuming and the storage and access of patient histories more reliable. The use of technicians to undertake routine tasks has facilitated the introduction of medication review services utilising pharmacists’ drug knowledge. Effective use of professional expertise and health resources should eliminate inefficiency and duplication of effort (1-3) in healthcare delivery. Prescribing requires knowledge of adverse effects, doses, optimal routes, drug-drug and drug-food interactions, pharmacokinetics, pharmacodynamics and monitoring of effects. Application of this knowledge requires significant expertise. (4)
Most medication-related interventions by pharmacists occur retrospectively; their earlier involvement in the prescribing process may help optimise the use of medicines. (5-7) Pharmacists’ interventions in medication management, including monitoring of therapy, are accepted in the hospital setting. (8) Extending this process by adding the right to prescribe (select) initial therapy and to adjust ongoing therapy is a relatively small step, and arguably, simply formalises a process that is already beginning.
There has been considerable discussion in the literature about the pros and cons of new professional practice models for pharmacists, including prescribing. It has been argued that pharmacists are developing expertise in evidence-based practice and patient-centred care, suiting them to taking responsibility for prescribing and monitoring therapy (2) with potential benefits for medical practitioners.
Over time, pharmacists have been prescribing an increasing range of medications. In many countries, the existence of ‘pharmacist only’ medicines recognises the expertise and competence of pharmacists to prescribe. (2) Further, in a number of countries, pharmacists are already able to legally prescribe a range of medicines previously only prescribed by medical practitioners. (9-11)
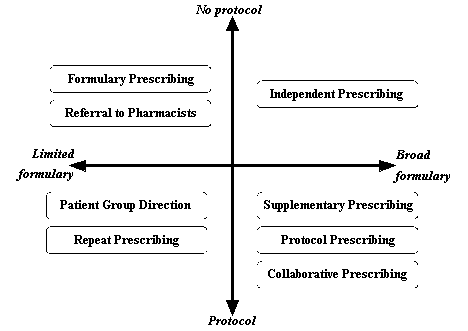
With this general acceptance of pharmacist prescribing in the international pharmacy literature, it is timely to investigate the implementation of pharmacist prescribing models internationally. These models are discussed below, categorised according to their degree of independence.
Independent prescribing
Independent prescribing occurs where the prescribing practitioner is solely responsible for patient assessment, diagnosis and clinical management (2) and requires legally defined levels of knowledge and skill that are usually monitored through a licensing process (2,12) . No examples of unrestricted independent pharmacist prescribing were identified in the literature.
Dependent prescribing: prescribing by protocol
‘Dependent’ prescribing incorporates more restriction on prescribing activities, via protocols or formularies. Prescribing by protocol is the most common form of dependent prescribing, (13) and is defined as “delegation of authority from an independent prescribing professional, usually a physician”, involving a formal agreement (protocol). (12) The protocol is a written guideline, (12) an explicit, detailed document that describes the activities pharmacists may perform in their prescriptive authority (12,13) .
The protocol lists:
· Types of diseases, drugs/drug categories (12,13) and prescriptive decisions covered by the agreement (7)
· The procedure, decision criteria or plan that the pharmacist must follow when prescribing (12,13)
· The physician and pharmacists party to the agreement (7)
· The time limit for the agreement (7)
· The responsibilities of each of the parties involved (12)
· The documentation required (7,12) and feedback mechanisms to the authorising prescriber (12)
· Policies for review and revision of the protocols. (12)
The level of authority should be determined by physicians’ assessments of the pharmacists’ competence, pharmacists’ assessment of their own competence, and pharmacists’ comfort with these roles (11) . Both parties should be willing to share responsibility for patient outcomes (12) .
Many drug groups have been deemed suitable for pharmacists’ prescribing by protocol, including anticoagulants (12,14,15) , analgesics (12,14,15) , antiemetics (12,15) and antihypertensives (11,12,14) .
Nurse prescribing under protocols, known as ‘standing orders’, is common in hospitals (16) . In New Zealand, any registered health professional can enter into ‘dependent’ prescribing arrangements with authorised prescribers under ‘standing orders’ or protocols (17) .
In the United States of America (USA), protocol-based prescribing had been successfully legislated in at least 25 states by 2001 (1,12) . The prescriptive authority requires prior state Board notification of the written protocol (7,11) . In the USA, Indian Health Service (IHS) pharmacists can prescribe for patients with disease states including ear infections, urinary tract infections, sexually transmitted diseases, congestive heart failure, hypertension, seizures, bacterial and fungal infections, arthritis and conjunctivitis (11) . In 1979, the IHS reported that physicians had judged the quality of pharmacists’ care as not significantly different to their own. The IHS model was also beneficial for patient satisfaction and pharmacist-physician relationships, reduction in physician referrals, and improvement in clinic efficiency (11) .
Credentialing is another issue addressed in this model. Local pharmacies were permitted to determine the scope of practice and establish the credentials needed for prescribing. Prescribing pharmacists generally required a PharmD or MS degree, or equivalent qualification, specialty board certification or two years of clinical experience (11) . The facility’s medical committee or chief executive officer then approved the scope of practice based upon competence, not educational attainment.
Policy changes included the application of formal written protocols and standing orders for prescribing, requiring legislative change. Quality assurance was provided by retrospective chart reviews by physicians.
It has been proposed that prescribing by protocol can lead to containment of drug costs (14) , reduction of medical practitioner visits, (18) integration with medication reviews, (18) and improving access to medicines, for example the emergency contraceptive pill. (14) In contrast, it may remove some interaction with the physician undertaking diagnosis (18) , create extra workload for the prescriber (18) , complicate reimbursement for prescribing, (14,18) require pharmacists to compromise other professional duties (17) and arguably lead to more room for error by involving more staff.
Implementation issues include coordination of information and access (17) , accreditation, education, accountability and competency assessment, (11,17) determination of scope of practice (17) and gaining of prescriptive authority (15) .
Dependent prescribing models: Patient Group Directions
A Patient Group Direction (PGD) is a written direction signed by a doctor or dentist, and by a pharmacist, relating only to supply and administration of a prescription medicine (1,19) . The recipients are any patients (1,19,20) , who may not be individually identified before presentation for treatment. (21) The PGD applies if a number of specified requirements are met (20) , subject to any specific exclusions listed (19) .
The PGD is a United Kingdom (UK) model that can be authorised by designated NHS bodies in the UK. (1,19) Trials have been reported in Manchester, two health authorities in London, South Derbyshire, Walsall and Bridgend (19) .
The PGD must specifically name the Prescription Only Medicine or class of medicines, dosage form(s), applicable dosage or maximum dosage, route of administration, frequency of dosing, minimum/maximum period for administration, relevant warnings, restrictions on quantity, circumstances in which the medicine can and cannot be supplied, when further advice should be sought, follow-up action, records to be kept, and the valid period for the PGD (19) .
Specific drugs listed in the literature for prescribing by PGD are emergency hormonal contraception, (19) combined oral contraceptives (22) and antihistamines (30-day courses) (23) . NHS-accredited pharmacists prescribing by PGD require dedicated training. Privacy during consultations remains an issue (22) . Pharmacists should be able to manage this service concurrently with their provision of ‘pharmacist only’ medicines (19) .
Dependent prescribing models: prescribing by formulary
In formulary-based prescribing, local formularies are agreed between participating medical practices and community pharmacies. (1) The formulary is a limited list of medicines (12,13) , including treatable symptoms, length of treatment, criteria for referrals and limitations for prescribing (23) . Many of the formulary medicines are those already available without prescription in a similar formulation or lower potency (24) . The model is less explicit than protocol prescribing (12,13) .
In the
A Scottish study of pharmacists’ prescribing by formulary included 11 therapeutic areas (23) . There was no evidence of abuse of the system in the Scottish trial, and the scheme was well received by patients (23) .
The model requires considerable record-keeping, and has been perceived to add liability to pharmacists (24) . Policy issues also include prevention of over-prescribing to patients consulting more than one pharmacist (23) . The optimal physical environment in the pharmacy should be determined such as an extra pharmacist and private consultation area (23) . If this scheme were widely adopted, a national pharmacy formulary would be recommended (23) .
Dependent prescribing models: prescribing by patient referral
Patients (1) , practice staff (1,12) or another community pharmacist (1) may refer patients to a pharmacist for a prescription. Typically, patients would be individually referred to a pharmacist by a physician for “management of specific drug therapy or to achieve a specific therapeutic outcome” (12,13) . The most common example of this model is the ambulatory care setting within a health care facility (12,13) .
A trial of patient referral to pharmacists (with formulary-guided prescribing) in Merseyside, UK, involved 12 minor ailments (26) . A single medical practice referred 38% of all presentations to one of eight pharmacies. Patients were less likely to accept referral if they perceived a need for a physician’s examination, if previous self-treatment had been unsuccessful, if the patient was a child, if the patient had a concomitant condition or influential medical history, or self-perceived a need for an antibiotic. In this study, physicians’ workload dealing with minor ailments decreased from 8.9% to 6.6% due to the referral system, and patients reported saving time and had improved accessibility to providers and treatments. The program costs were “not substantial”, and overall prescribing costs did not increase. The referral model involved identification of eligible ailments at the medical practice and offering pharmacists’ consultation. A consultation form was completed by reception staff and faxed to the chosen pharmacy, where the pharmacist recommended a medicine from the formulary or referral back to a medical practitioner. Pharmacists were paid £1.50 for their professional input, while medicines were supplied under NHS subsidy.
Dependent prescribing models: repeat prescribing
Repeat prescribing involves pharmacists providing medication-refill services in clinics associated with medical centres, for patients who have exhausted their prescribed drugs before their next physician appointment. The pharmacist assesses the patient and therapy and either:
· Consults the attending physician if there are problems with compliance, disease control and/or side effects (11,27)
· Writes refill prescriptions for dispensing at another pharmacy (14) or
· Refills the medication with a sufficient quantity to last until the next available appointment. (11,27)
Repeat prescribing has been discussed in the UK (28) , and is allowed in some 28 states in the USA (14) , although there is a paucity of literature on repeat prescribing trials. In the Australian system, the Pharmaceutical Benefits Scheme has in place a procedure for repeat prescriptions written by medical practitioners.
Dependent prescribing models: supplementary prescribing
Supplementary prescribing is a voluntary partnership between the independent prescriber and a supplementary prescriber, to implement an agreed patient-specific clinical management plan with the patient’s agreement (1,21,29) . No clinical situations are listed in the definition, to avoid excluding others (1,21) . Supplementary prescribing is not restricted to one-to-one prescriber partnerships, as patient care is largely delivered by teams (1) .
The independent prescribers are doctors or dentists (29,30) . Supplementary prescribers are registered pharmacists or nurses (1,21,29,30) . The independent prescriber undertakes the initial assessment (21,29) and the supplementary prescriber can then write prescriptions at public expense (1,29) , working to a care management strategy that has been agreed by the physician (31) .
The supplementary prescriber’s roles include contributing to clinical management plan monitoring (1) , changing the medicine and referring to the independent prescriber where appropriate (1) , and recording clinically relevant facts (1,29) .
Central to the prescribing arrangement is the patient-specific clinical management plan that is evidence-based, consistent with recognised clinical guidelines, and agreed by prescribers and the patient or carer (1) . Patients are involved in decision-making (29) and provide consent for the transfer of their information between prescribers (1) . Prescribing and dispensing should be separate for patient safety and governance (1) , if not, clear accountability arrangements should be in place (29) .
There is no restriction on the medical conditions to which this model applies (1,21) , but supplementary prescribing is unlikely to be used for acute conditions (29) . All medicines, excluding controlled drugs (21,29) and unlicensed medicines (21) may be prescribed.
In the
Although evaluation is ongoing, supplementary prescribing is expected to demonstrate multiple benefits for health care delivery and organization (1) , patient convenience (1,32) , access (1,21,33) , patient safety (21) , concordance with clinical management plans (1) , efficiency in general practice and hospitals (1) , waste reduction (1) , doctors’ workload (21,32,33) and professional satisfaction for pharmacists (1) .
Collaborative prescribing models
Collaborative prescribing requires a cooperative practice relationship between a pharmacist and a physician or practice group, with legal authority to prescribe medications (12) . Explicit collaborative agreements are negotiated within each facility (12) , outlining who is delegating and receiving authority, and demonstration of competence. The group of patients may be defined by the pharmacist’s expertise. In the USA, agreements must be filed with a State Pharmacy or Medical Board (2) .
The physician diagnoses and makes initial treatment decisions for the patient, and the pharmacist selects, initiates, monitors, modifies and continues or discontinues pharmacotherapy as appropriate to achieve the agreed patient outcomes. The physician and pharmacist share the risk and responsibility for the patient outcomes (13) .
Informally, clinical pharmacists in public and private hospitals have practised collaborative prescribing to some extent throughout the past 25 years (8,20,34-37) . Examples of collaborative prescribing by hospital pharmacists in Canada and the USA include aminoglycoside and pharmacokinetic dosing services, anticoagulant therapy adjustment and chemotherapy antiemetic management (12) . By 2001, 27 American States had some form of legislation that allowed collaborative practice between pharmacists and physicians (38) . In Minnesota, pharmacists may provide medicines for first dosages and in emergencies (39) . Collaborative models are being considered in Canada for pharmacists’ prescribing (13,40) , including initial drug selection or adjustments (12) .
A study has been undertaken to demonstrate the appropriateness of prescribing and monitoring by hospital pharmacists (20) . Evidence also supports that provision of cognitive services by community pharmacists improves patient health outcomes and possibly reduces health care costs (41) .
An Australian hospital study found that a significant proportion of doctors would welcome pharmacists making written comments in the medical record notes (42,43) . UK surveys, however, indicate that pharmacists, doctors and nurses have mixed feelings about the introduction of expanded roles for pharmacists (20,44,45) .
Implementation issues
The eight models
identified from the
Despite the numerous commentaries in support of pharmacists’ prescribing, there is a lack of evidence about the impact of such models on practice and outcomes. There is potential for such models to be implemented in a rapidly changing health care system as the fundamental principles of patient-centered and integrated care in a financially and clinically responsible manner apply. There are many issues that must be addressed before such models can be implemented; these are discussed below.
1. Professional issues
Responsibility must be taken for the whole process of diagnosis, prescribing and follow-up, including an awareness of boundaries or limitations to expertise (46) . Not all pharmacists may want to undertake this responsibility.
There is a need for appropriate baseline and continuing professional education (12,46) . It is probable that all registered pharmacists have the expertise required to undertake dependent prescribing without further intensive education, other than training in the prescribing process itself (2,3) . It is important, however, that all pharmacists undertaking independent prescribing roles demonstrate competence, and that a register be kept of suitably qualified pharmacists (3) . Collaborative prescribing may only require the assessment of competence at an institutional level (12) . A contrasting viewpoint is the need for a uniform standard of competence for prescribing pharmacists, irrespective of the prescribing model utilised (9,47) .
The separation of dispensing and prescribing is seen as an important component of the ‘checks and balances’ ensuring that the most appropriate treatment is chosen for the patient. Pharmacists currently prescribe and dispense for minor conditions; however, extending this responsibility to prescription medicines may introduce new quality assurance issues.
Periodic review of pharmacists’ prescribing practices may be required as a mechanism for maintaining standards and ensuring optimal patient outcomes (3,11) . Audits may focus on such activities as adherence to prescribing protocols, adverse events or outcomes assessment (48) .
Maintenance of, and access to, patient records are required when a pharmacist prescribes medication. The system should be comprehensive, effective and time efficient (48,49) , and may require transfer of information back to the medical practitioner.
Securing remuneration for professional responsibility is another essential step in the adoption of prescribing rights. Pharmacists who have undertaken additional education and are prepared for the increased responsibility associated with prescribing should be appropriately compensated financially for this task (6,12) . There are few models of remuneration available, and their international applicability is questionable.
2. Changes to medication prescribing
International developments show that enhanced clinical roles for pharmacists are valuable, and that pharmacists have the expertise to contribute to patient care. For widespread acceptance, services provided by pharmacists must be promoted to the public, health care system administrators and government (50) . Pharmacist prescribers must also recognise the rights of consumers to choose the practitioner of their choice and to reject recommendations that are made (48) .
There may be resistance to change from within the pharmacy profession, and other professions may feel that prescribing pharmacists intrude on their area of professional responsibility (46) . The careful development of collegial working relationships is essential in the acceptance of new prescribers.
With successful implementation of alternative prescribing models, costs for patients may decrease (46) , as knowledge of the cost associated with therapeutic alternatives may inform treatment decisions. Costs for pharmacists may increase if the physical layout of the pharmacy requires modification to facilitate private counselling (3) .
 |
3. Workload and workforce issues
There are workforce-related issues related to pharmacists gaining prescribing rights. It may be possible that pharmacists who prescribe medicines spend less time seeking approval for changes to existing prescriptions or obtaining ‘owed’ prescriptions, therefore improving workload. Adopting and maintaining new services may present a challenge for the pharmacy profession in some countries where a shortfall in pharmacists has been predicted (50,51) .
4. Legal issues
For successful implementation of pharmacist prescribing models, a statutory framework needs to be in place, and a separate register of those pharmacists who have been judged competent to practise as ‘prescribing pharmacists’ is recommended (52) . Legal requirements may depend on the actual model implemented, considering the degree of responsibility required of the pharmacist.
CONCLUSION
There are many papers that voice opinion and rhetoric about pharmacists’ prescribing of Prescription Medicines. From the literature, we identified eight relevant models implemented internationally. The impact of these models on health outcomes and health care systems have not been well studied.
If pharmacists are to be granted the right to prescribe, they must also accept the inherent responsibilities. Establishing a rigorous clinical governance framework will be critical to establishing prescribing models in any setting. There are numerous professional, technological, educational and legal issues that must be resolved before pharmacists can prescribe. The introduction of collaborative or supplementary prescribing models may be an appropriate first step.
ACKNOWLEDGEMENT
The authors wish to acknowledge research funding from the Australian Commonwealth Department of Health and Ageing as part of the Third Community Pharmacy Agreement.
REFERENCES
(1) Crown, J., Report of the Review of Prescribing, Supply and Administration of Medicines. House of Commons, London, UK, 1999.
(2) Galt, K., The key to pharmacist prescribing: collaboration. Am J Health Syst Pharm, 52:1696-1699, 1995.
(3) Stoate, H., Pharmacist Prescribing. A Report to Health Ministers. House of Commons, London, UK, 2001.
(4) Anderson, S., The state of the world's pharmacy: a portrait of the pharmacy profession. J Interprof Care, 16:391-404, 2002.
(5) Davies, J., Horne, R., Bennett, J. and Stott, R., Doctors, pharmacists and the prescribing process. Br J Hosp Med, 52:167-170, 1994.
(6) Hindmarsh, K., Optimal drug therapy: the role of the pharmacist in bridging the gap between knowledge and action. Can J Clin Pharmacol, 8 Suppl A:53A-54A, 2001.
(7) Carmichael, J. and Cichowlas, J., The changing role of pharmacy practice--a clinical perspective. Spec Law Dig Health Care Law, 9-20, 2002.
(8) Galindo, C., Olive, M., Lacasa, C., Martinez, J., Roure, C., Llado, M., Romero, I. and Vila, A., Pharmaceutical care: pharmacy involvement in prescribing in an acute-care hospital. Pharm World Sci, 25:56-64, 2003.
(9) Pharasi, B., Price, M. and Goldstein, S., Act 101 and the pharmacist--is it appropriate for the pharmacist to prescribe? S Afr Med J, 83:822-823, 1993.
(10) Curtiss, F., Crossing the Quality Chasm-Pharmacist Prescribing, Nontraditional Interventions, and Outcomes-based Pharmacist Reimbursement (OBPR) [In Process Citation]. J Manag Care Pharm, 8:403-4, 2002.
(11) Herrier, R., Boyce, R. and Apgar, D., Pharmacist-managed patient-care services and prescriptive authority in the U.S. Public Health Service. Hosp Formul, 25:67-8, 76-8, 80, 1990.
(12) Task force on Pharmacist Prescribing (Pearson, G., Card, D., Chin, T., Gray, M., Hawboldt, J., Jackevicius, C., Slavic, R., and Thompson, A.), An Information Paper on Pharmacist Prescribing within a Health Care Facility. Canadian Society of Hospital Pharmacists, Canada, 2001.
(13) Pearson, G. J., Pharmacist Prescribing. Taking on a new role - and new responsibilities. Pharm Connects, November/December, 1998.
(14) Chi, J., Pharmacist prescribing is advancing slowly but surely throughout the country. Drug Topics, 20:39, 2000.
(15) Fuller, T., Collaborative Drug Therapy Management: Concept and Application. University of Washington, Washington, 2003.
(16) NZ Ministry of Health, Consultation Document: Nurse Prescribing in Aged Care and Child Family Health. NZ Ministry of Health, Wellington, New Zealand, 1998.
(17) Shaw, J., Pharmacist Prescribing. Pharmaceutical Society of New Zealand, Wellington, New Zealand, 2002.
(18) Aldous, J., Hospital pharmacist perspective: prescribing pharmacists. Information to Pharmacists (serial online), 2003. Available from: http://www.computachem.com.au/i2P/emag/Issue17/Article10.shtml. Accessed Oct 18, 2004.
(19) Anonymous, The locum report: emergency hormonal contraception. The Locum, Spring:8-9, 2001.
(20) Child, D., Hospital nurses' perceptions of pharmacist prescribing. Br J Nurs, 10:48-54, 2001.
(21) Shaw, N., Pharmacist Prescribing: the UK-NHS Approach. Society of Hospital Pharmacists of Australia (Qld Branch), Toowoomba, Australia, 2004.
(22) Taylor, B., The role of the pharmacist in emergency contraception. J Fam Plann Reprod Health Care, 29:7, 2003.
(23) Duff, N., The direct supply of medicines pilot in Scotland - a local view. Pharm J, 270:26, 2003.
(24) Savino, T., Legal Authority for a Pharmacist's Order for Prescription Drugs, WebRx Pharmacy Palace, 2002. Available from: http://www.rxpalace.com/laws.htm. Accessed Oct 18, 2004.
(25) Granby, T., Lessons learnt from the nurse prescribing experience. Pharm J, 270:24, 2003.
(26) Anonymous, Care at the Chemist: a question of access. School of Pharmacy and Pharmaceutical Sciences, University of Manchester, UK, 2001.
(27) Cram, D., Stebbins, M., Eom, H., Ratto, N. and Sugiyama, D., Peer review as a quality assurance mechanism in three pharmacist-run medication-refill clinics. Am J Hosp Pharm, 49:2727-2730, 1992.
(28) Anonymous, Prescribing rights extended in UK. Can Med Assoc J, 168:209, 2003.
(29) Root, G., Supplementary prescribing - a groundbreaking opportunity. Pharm J, 270:19-20, 2003.
(30) Mullan, K., Supplementary prescribing and access to medical records: (1) Existing duties and responsibilities. Pharm J, 270:902-903, 2003.
(31) Hilton, L., The story behind enhanced nurse and pharmacist prescribing, Part II. News Release 8 May 2002. HealthCare Hub, 2002. Available from: http://uk.healthcarehub.com/News/Features/article.cfm?AID=129. Accessed Dec 31, 2002.
(32) Alliance NHS, Proposals to extend prescribing powers. In: Health and Community Care. News Release 17 April 2002. Available from: http://www.scotland.gov.uk/pages/news/2002/04/SEhd013.aspx. Accessed Oct 18, 2004.
(33) Hall, J., Can we learn from nurse prescribing? Pharm J, 272:56, 2004.
(34) Leape, L., Cullen, D., Clapp, M., Burdick, E., Demonaco, H., Erickson, J. and Bates, D., Pharmacist participation on physician rounds and adverse drug events in the intensive care unit. JAMA, 282:267-270, 1999.
(35) Thornton, J., Goff, D., Segal, R. and Guy, J., Impact of a clinical pharmacist on antibiotic prescribing. A multicenter trial. J Pharm Technol, 7:195-200, 1991.
(36) Furmaga, E., Pharmacist management of a hyperlipidemia clinic. Am J Hosp Pharm, 50:91-95, 1993.
(37) Boddy, C., Pharmacist involvement with warfarin dosing for inpatients. Pharm World Sci, 23:31-35, 2001.
(38) Huff, P., Pharmacist prescribing through collaborative practice agreements. Almanac Alliance for CME, 23:3, 2001.
(39) Keely, J., Pharmacist scope of practice. Ann Intern Med, 136:79-85, 2002.
(40) Gray, M., Pharmacist prescribing: striving for excellence in patient care. Can J Hosp Pharm, 55:91-92, 2002.
(41) Farris, K., Kumbera, P., Halterman, T. and Fang, G., Outcomes-based pharmacist reimbursement: reimbursing pharmacists for cognitive services, part 1 [In Process Citation]. J Manag Care Pharm, 8:383-393, 2002.
(42) Clifford, R. M., Jessop, J. B. and Lake, J., Evaluation of clinical pharmacy services: a survey of doctors' opinions. Aust J Hosp Pharm, 23:11-16, 1993.
(43) Dooley, M., Allen, K. M., Doecke, C. J., Galbraith, K. J., Taylor, R. J., Bright, J. and Carey, D. L., A prospective multicentre study of pharmacist initiated changes to drug therapy and patient management in acute care government funded hospitals. Br J Clin Pharmacol, 57:513-521, 2003.
(44) Spencer, J. and Edwards, C., Pharmacy beyond the dispensary: general practitioners' views. BMJ, 304:1670-1672, 1992.
(45) Edmunds, J. and Calnan, M., The reprofessionalisation of community pharmacy? An exploration of attitudes to extended roles for community pharmacists amongst pharmacists and General Practitioners in the United Kingdom. Soc Sci Med, 53:943-955, 2001.
(46) Wilhelmsson, S. and Foldevi, M., Exploring views on Swedish district nurses' prescribing - a focus group study in primary health care [In Process Citation]. J Clin Nurs, 12:643-650, 2003.
(47) Bacon, L. and Savage, I., Training and supporting pharmacists to supply progestogen-only emergency contraception. J Fam Plann Reprod Health Care, 29:17-22, 2003.
(48) Newdick, C., Pharmacist prescribing - new rights, new responsibilities. Pharm J, 270:25, 2003.
(49) Gray, D., Documentation and assessment of pharmacist-initiated drug therapy interventions. Top Hosp Pharm Manage, 11:69-79, 1992.
(50) Anonymous, A vision of pharmacy's future roles, responsibilities, and manpower needs in the United States. American College of Clinical Pharmacy. Pharmacother, 20:991-1020, 2000.
(51) Health Care Intelligence Pty Ltd, A Study of the Demand and Supply of Pharmacists, 2000–2010. The Pharmacy Guild of Australia, Canberra, Australia, 2003.
(52) Purcell, K., Pharmacy as a medical specialty. Am J Health Syst Pharm, 60:954; author reply 954-5, 2003.
Corresponding
author: Dr Lynne Emmerton,
Published by the Canadian Society for Pharmaceutical Sciences.
Copyright © 1998 by the Canadian
Society for Pharmaceutical Sciences.
http://www.cspscanada.org