J Pharm Pharmaceut Sci (www.cspscanada.org) 8(2):299-315, 2005
A topical w/o/w multiple emulsions prepared with Tetronic 908 as a hydrophilic surfactant: Formulation, characterization and release study
Figen TIRNAKSIZ, Ozlem KALSIN. Gazi University Faculty of Pharmacy, Department of Pharmaceutical Technology, Etiler – 06330, Ankara, Turkey.
Corresponding author: Figen TIRNAKSIZ, Gazi University Faculty of Pharmacy, Department of Pharmaceutical Technology, Etiler – 06330, Ankara, Turkey. e-mail: feegosh@yahoo.com
Received February 14, 2005, Revised May 25, 2005, Accepted June 6, 2005, Published August 11 2005
ABSTRACT
Purpose: The aim of this
work was to prepare the stable water/oil/water multiple emulsions (MEs), to
investigate the usage of poloxamine 908, to observe the influence of surfactant
percentage on the properties of MEs. Method:
MEs were prepared by liquid paraffin, cetyl dimethicone copolyol and poloxamine
908 by a two-step emulsification procedure. Caffeine was used as a water-soluble
model. The viscosity, conductivity and globule size of MEs were followed over
time. Results: The formulations containing 1% cetyl dimethicone copolyol
and 1 or 2% poloxamine 908 were the most stable systems. The globule size of
MEs ranged from 20 to 37 µm and did not change during time. The yield of MEs was between 99.6% and 98.7%.
The conductivity increased and the viscosity of systems decreased during time.
Increase in poloxamine 908 influenced the viscosity of the system, with the
viscosity decreasing as the hydrophilic surfactant concentrations were
increased. Caffeine release from the MEs was slow; the release was affected by
both surfactant concentrations. Conclusion:
Poloxamine 908 could be used as a hydrophilic surfactant for formulation of
w/o/w MEs. The concentration of poloxamine 908 was a very important parameter
in preparing stable MEs. It was
concluded that caffeine might be transported out by molecular diffusion and
through a reverse micellar mechanism controlled by the viscosity of the system
INTRODUCTION
Multiple emulsions are vesicular and
complex systems [1]. They can be considered as emulsions of emulsions and have
shown promise in cosmetic [2, 3], pharmaceutical and separation sciences [4].
Their potential pharmaceutical applications
include uses such as taste masking, adjuvant vaccines, an immobilization of
enzymes and sorbent reservoir of overdose treatments, and for enhancement of
enteral or dermal absorption [5-8]. Multiple emulsions have been formulated as
cosmetics, such as skin moisturizer [9]. Prolonged release can also be obtained
by means of multiple structures [10-13]. These systems have some advantages,
such as the protection of the entrapped substances [14, 15] and the
incorporation of several actives in the different compartments [16]. Despite
their potential usefulness, applications of multiple emulsions have been
limited because of thermodynamic instability and their complex structure.
Water/oil/water (w/o/w) multiple emulsions
consist of dispersed oil globules containing smaller aqueous droplets; each
inner aqueous droplet is separated from the outer aqueous phase by an oil phase
layer. The presence of at least two surfactants is required. One of them is
predominantly lipophilic for stabilizing the primary water/oil (w/o) emulsion
and the other is hydrophilic for the secondary oil/water emulsion. To produce a
w/o/w emulsion, the lipophilic and hydrophilic surfactants are dissolved in oil
and continuous aqueous phase, respectively. The most common preparations of
w/o/w double emulsions are based on the two-step emulsification process. The
stability and release characteristics of multiple emulsions are influenced by
different factors, such as surfactant type, surfactant ratio and some physical
properties of the system (globule size, viscosity, conductivity, phase volume
ratio, etc) [1, 17, 18].
The Poloxamine 908 (Tetronic®908)
used in this study as a hydrophilic surfactant is nonionic and a block
copolymer [19]. We could not find any published work on the usage of the
Tetronic®908 in the formulation of multiple emulsions. Cetyl
dimethicone copolyol (Abil®EM90) used as a lipophilic surfactant is
the siliconic polymeric surfactant, and shows good trapping capacity, prolongs
the release of active molecules, and produces a w/o emulsion with strong
interfacial film [4, 20, 21].
The purposes of this research were i) to investigate the usage of Tetronic®908
for w/o/w multiple emulsion formulation, ii)
to evaluate the formation and stability of the multiple emulsion system, iii) to investigate the properties of the
system and to observe the influence of hydrophilic and lipophilic surfactant
percentage on the characteristic properties of multiple emulsions.
EXPERIMENTAL
Chemicals
The following substances were used for the
preparation of multiple w/o/w emulsions: The oil used was liquid paraffin
(η: 110-230 mPa.s, Merck). Abil®EM90
(cetyl dimethicone copolyol,
Caffeine (Merck) frequently used in
cosmetic products was selected as a hydrophilic model substance for release
experiment. Sodium chloride (NaCl) was incorporated into the aqueous internal
phase and used as a conductimetric tracer.
All materials were of analytical grade and
used without further purification.
Preparation
of w/o/w multiple emulsion
Multiple emulsions were prepared by a
two-step emulsification process [2]. The first emulsification was to prepare
the w/o primary emulsion and the second emulsification step provided the
formation of the w/o/w multiple globules. The compositions of formulations are
shown in Table 1.
Electrolyte or both electrolyte and
caffeine were dissolved merely in the aqueous phase of the primary w/o
emulsion. The osmotic pressure of the internal aqueous phase of multiple
emulsions containing or not-containing caffeine was adjusted to 3.4 atm.
The concentrations of paraffin oil and
electrolyte in primary emulsions were fixed at 30 and 0.3%, respectively. The
percentage of the primary emulsion was also constant at 80%.
|
Table 1: Composition of the w/o/w multiple emulsions |
|
|
First emulsification (for primary w/o emulsion) |
|
|
Without caffeine Oil phase |
|
|
Liquid paraffin |
30% |
|
Abil®EM90 |
2 or 4% |
|
Internal aqueous phase |
|
|
Sodium chloride |
0.3% |
|
Distilled water to |
100 |
|
With caffeine Oil phase |
|
|
Liquid paraffin |
30% |
|
Abil®EM90 |
2 or 4% |
|
Internal aqueous phase |
|
|
Sodium chloride |
0.03% |
|
Caffeine |
1.5% |
|
Distilled water to |
100 |
|
Second emulsification (for w/o/w multiple emulsion)* |
|
|
Oil phase |
|
|
Primary w/o emulsion |
80% |
|
External aqueous phase |
|
|
Tetronic®908 |
1, 2 or 4% |
|
Distilled water to |
100 |
|
*The caffeine concentration
of multiple emulsions = 1.2% |
|
Water/oil primary emulsions were prepared
with different concentration of a lipophilic surfactant (Abil®EM90). Two concentration of
Abil®EM90 were used in
the primary emulsion (2 and 4%). The oily mixture of Abil®EM90 and paraffin was heated to 75oC and
then aqueous solution at the same temperature was added to this mixture by
stirring at 1500 rpm at 75 oC for 30 minutes. The first step was
carried out using the mechanical mixer to produce fine droplets. The emulsion
prepared was then cooled to room temperature. The mixer used to prepare the MEs
was the similar of Microvortex mechanical mixer (Granier Charvet SA, France)
and it was produced in
The stability of the w/o primary emulsion
prepared at the first emulsification was an important factor determining the
stability of the w/o/w emulsion. Therefore, the accelerated physical stability
of primary emulsions was tested under centrifugation (Mechanica Precyzynjna,
Warszawa, MPW-340, Poland) during 1 hour at 3500 rpm (10060 m/s2 or
1026 g), and the occurrence of phase
separation was examined. The stability of w/o emulsions under centrifugation
reflects the strength of the interfacial film between the aqueous and oil
phases. No change was macroscopically observed in any of the primary emulsions
in this study, which were quite stable. Moreover, no phase separation was
observed during 6 months at room temperature.
Water/oil/water multiple emulsions
containing caffeine were prepared by the same emulsification procedure under
the same conditions. Both caffeine and NaCl were dissolved into the inner
aqueous phase. The osmotic pressure created by adding caffeine was calculated
according to Van’t Hoff’s equation, and NaCl concentration was decreased to
equilibrate the osmotic pressure increased by the presence of caffeine in the
internal droplets.
For the preparation of the w/o/w multiple
emulsion, the freshly prepared w/o primary emulsion (80g) was emulsified
further in an external aqueous phase (20 g) inside which the hydrophilic
surfactant was dissolved at room temperature. The mixture (100 g) was stirred
using mechanical mixer at
The formation times of multiple globules
were determined, and this experiment was done with multiple emulsions
containing caffeine. The caffeine concentration of MEs was 1.2%.
Characteristics
of multiple emulsions
After preparation of the multiple emulsions
not containing caffeine, several physical tests were carried out in order to
determine their characteristics, such as viscosity, mean multiple globule size
and conductivity.
In this study, the physical stability of
w/o/w multiple emulsions prepared was also examined using viscometric,
conductimetric and granulometric methods. In addition, formulations were
evaluated by visual observation of the phase separation. Macroscopic analysis
was carried out to observe the homogeneity of the systems.
The multiple emulsions were stored at 25
and 40 oC, and the characteristic properties of the systems
were followed until phase separation was observed. At a definite time, the
emulsions were allowed to return to room temperature before observation, and
then the viscosity, mean multiple globule size and conductivity values were
determined.
The data were evaluated by statistical
analysis according to the Student’s-t test. In addition, standard error (SE)
was computed for every mean value.
Microscopic
analysis, measurement of globule and droplet size
Microscopic analysis was carried out using
an optical immersion microscope (Soif, Model 4GX, AN-KA, Istanbul-Turkey), and
observations were made at 100x16 magnification after diluting in the
appropriate external phase of emulsion. This examination provided direct
information on the multiple structures.
We could see the internal aqueous phases as droplets in a w/o/w emulsion
structure. The existence of multiple
globules was checked microscopically during all experiments until the phase
separation was observed.
Granulometric analysis was carried out in
order to characterize the globule size of multiple emulsions and the droplet
size of w/o primary emulsion. The mean size of dispersion phase for every
emulsion was determined with a laser diffraction particle sizer (Sympatec GmbH,
HELOS Particle Size Analysis,
|
Table 2: Main properties of multiple emulsions
after immediately preparation |
||||
|
Abil
EM90 |
Tetronic
908 |
Viscosity* at 5 rpm (mPa.s) |
Globule
size (µm) |
Conductivity (µS) |
|
2% |
1% |
60000 |
20.6 |
21.3 |
|
2% |
2% |
58000 |
20.6 |
26.8 |
|
2% |
4% |
46000 |
34.6 |
28.4 |
|
4% |
1% |
60000 |
29.5 |
28.9 |
|
4% |
2% |
54000 |
30.2 |
53.9 |
|
4% |
4% |
44000 |
36.8 |
64.0 |
|
|
|
|
|
|
Measurement
of viscosity
For the viscometric measurements, the
samples of multiple emulsions were examined using Brookfield LV rotational
viscometer (Brookfield Engineering Lab. Inc. MA,
Conductimetric
analysis
The conductivity was measured with
conductimeter (HI-9033, Hanna Inst.). It was necessary to dilute multiple
emulsions with water or iso-osmotic NaCl solution to measure conductivity. The
preliminary studies were performed to establish whether further dilution could
influence the measurement. The measurements were made at room temperature on
samples of the multiple emulsion diluted
The entrapment percent or yield value (E%) was calculated according to the following
equation:
E%=
![]()
where Ci
is the conductivity of the internal aqueous phase (0.3% NaCl solution, 4800 µS)
and Ct is the conductivity
value of multiple emulsion at a given time t.
In
vitro release studies
Release study of caffeine from multiple
emulsions was investigated by a dialysis method. Before this experiment, the
saturation solubility of caffeine in buffer solution (pH 5.2) at 32 oC
was determined in triplicate. An excess amount of caffeine was added into
Dialysis tubing (seamless, D-0405,
lot:01H0713, Sigma) was used as a membrane for the release study and was washed
several times with distilled water and left soaking in buffer solution (pH 5.2)
overnight before use. Immediately after preparation, 2 g of the multiple
emulsions containing 1.2% caffeine was introduced into the dialysis tubing
double-tied at each end and dialyzed in
RESULTS
and DISCUSSION
In the development of w/o/w multiple
emulsions, it is necessary to estimate their physical stability experimentally.
Viscosity, multiple globule size and conductivity measurements are convenient
methods for this purpose [22].
In this study, we tried to formulate a
stable multiple emulsion with Tetronic®908 in the external phase as
the hydrophilic surfactant and with Abil®EM90 in the inner aqueous
phase as the hydrophobic surfactant. Two different concentrations of the
lipophilic surfactant and three different concentrations of the hydrophilic
surfactant were used in order to evaluate the influence of the surfactant
percentage on the characteristics of multiple emulsions (Table 1).
It was shown that the stability of the w/o
emulsion requires the presence of electrolyte in the aqueous phase; it is well
known that the electrolyte concentration or osmotic pressure of the internal
aqueous phase of w/o/w emulsions plays a critical role in the physical
stability of the system [23]. Thus, the optimum balance should be ensured
between the internal and external aqueous phase of w/o/w multiple emulsions
[24, 25]. Therefore, we examined the literature regarding the formulation of
w/o/w multiple emulsions, and it was decided to use NaCl at a concentration of
0.3% (0.0513 molar) as electrolyte [26].
Viscosity, mean globule size and
conductivity were determined to investigate the characteristics of multiple
emulsions not containing caffeine as a function of time. Measurements were made
up to five months at monthly intervals following preparation of the emulsions.
In addition, phase separation and phase inversion of multiple emulsions were
also investigated, and the results of visual observations are summarized in
Table 3. The beginning of phase separation is shown, which revealed a
destruction of multiple globules of the system. During storage time, no phase
inversion was observed in any multiple emulsions.
It is well known that coalescence is
amplified with temperature. In this study, the beginning of phase separation at
40 oC occurred earlier than at 25 oC, except for
formulations containing 4% Abil®EM90. For example, phase separation
was observed at the fourth month for the multiple emulsion containing 2% Abil®EM90
at 40 oC, whereas phase separation was observed at the fifth month
at 25 oC.
When the physical stability of multiple
emulsions containing varying amounts of Tetronic®908 and a constant
of Abil®EM90 was evaluated, it was observed that the stability
decreased as Tetronic®908 concentration increased. At high Tetronic®908 concentration (4%) coalescence occurred, resulting in
phase separation within three or four months. On the contrary, no phase
separation or physical stability problem occurred over five months at 25oC
for multiple emulsions with prepared lower concentrations of Tetronic®908
(1 and 2%). A similar finding was reported in other investigations [27-31].
Matsumota et al. [27] explained the effect of an increase in the amount of
hydrophilic surfactant on the physical stability of multiple emulsions as: During
the second emulsification, some of the molecules of the hydrophobic surfactant
from the oil layer may be solubilized in the external aqueous phase due to
amount of hydrophilic surfactant that is above critical concentration. This
phenomenon may be a very important cause of the rupture of the oil layer
covering the internal aqueous phase droplets after preparation of the system.
Microscopic
and globule size analysis
Microscopic technique is the useful direct
method to assess the formation and to follow the stability of multiple
globules. The various stability problems, such as globule deformation and phase
inversion to simple emulsion, may be detected using this method.
All multiple emulsions were prepared
successfully using the method described. The structure of w/o/w multiple
emulsions was observed under the microscope during
manufacturing. Many multiple globules were observed very well and many small
droplets were seen in the internal phase of multiple globules. The microscopic
view of a multiple emulsion is shown in Figure 1.
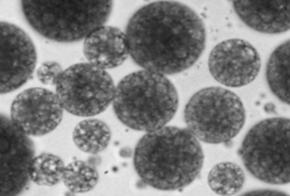
Figure
1: Microscopic view of multiple emulsion (100x16 magnification).
To evaluate the type of multiple emulsions,
a drop of a water-soluble dye (methylene blue) solution was added to the system
and dissolution of dye solution was observed. The colored mixture was then
examined by optical microscopy. This procedure revealed that water was the
continuous phase and that the sample was a w/o/w emulsion.
The size of globules is the most important
parameter in rheology and physical stability of any emulsion. The globule size
of multiple emulsions as a function of time is shown in Figure 2. When change in multiple globule size was investigated, no
significant increase or decrease was observed in the mean size during storage
time at 25 and 40 oC; this may not be evidence of non-coalescence. A similar result was also reported by
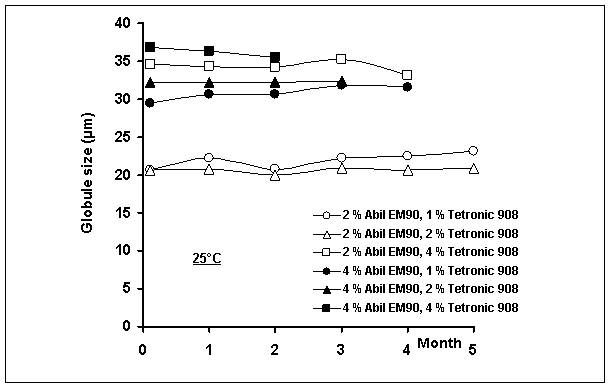
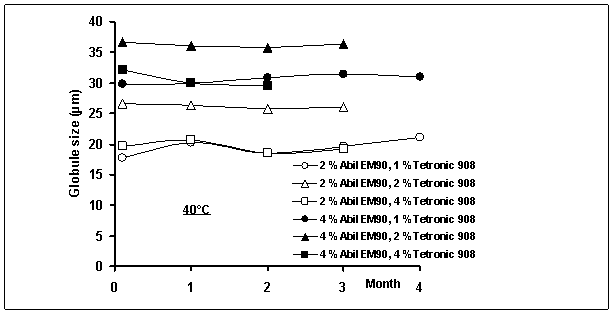
Figure
2: Globule size of multiple emulsions as a
function of time
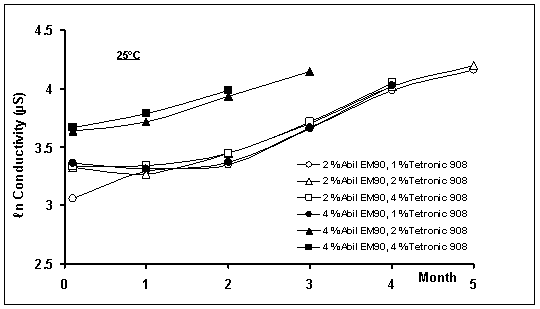
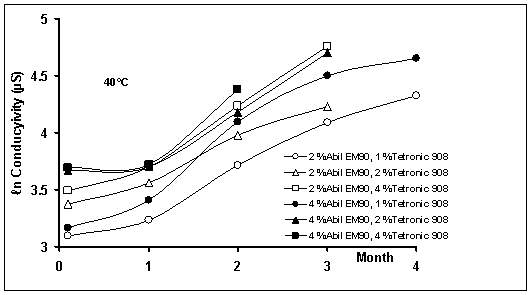
Figure
3: Effect of temperature on conductivity of w/o/w
multiple emulsions during storage time
The mean multiple globule sizes of
emulsions were recorded in the range of 20±0.025 µm and 37±0.031 µm (mean±confidence interval). In our previous study, the particle
size measurements of w/o primary emulsions demonstrated a mean droplet size
(±CI) of the aqueous phase, 3.38±0.0216 µm and 3.44±0.0541 µm for emulsions
prepared with 2% Abil®EM90 and 4% Abil®EM90, respectively
[34]. These values were statistically the same (p>0.05). This result showed that
there was no significant effect of high lipophilic amount (4%) on the
interfacial tension between water and oil phase; the effective reduction of
interfacial tension was probably ensured by 2% of Abil®EM90. The
globule size of primary emulsions did not change, and no visual sign of
physical destabilization was observed in systems at 25 and 45 oC
during storage time.
As expected, the mean globule size of
multiple emulsions depended on the surfactant concentrations.
When the effect of Tetronic®908
concentration on globule size of multiple emulsions was investigated, a small
increase with increasing surfactant amount was observed; the higher
concentration of hydrophilic surfactant most likely caused the coalescence of
globules due to rupture of oil layer between two aqueous phases during
manufacturing [27]. However, there was no significant effect of Tetronic®908
concentration (for low concentration, 1 and 2%) on globule size of multiple
emulsions containing the same percentage of Abil®EM90 (Table 2)
(p>0.05).
When the percentage of Abil®EM90
was increased from 2% to 4%, the globule size logically increased. The excess
of Abil®EM90 was located in a molecular form in the oil phase
between external and internal aqueous phase, and this caused swelling of the
oil globules (Table 2). On the contrary, the globule size of multiple emulsions
containing caffeine decreased with increasing Abil®EM90 percentage
and the same concentration of hydrophilic surfactant (Table 4). It was
concluded that caffeine influenced the tension of the second interface during
the second emulsification step.
Conductimetric analysis
This analysis was carried out in order to
directly measure the entrapped electrolyte within the internal aqueous phase
and to confirm the emulsion type. Conductivity data generated good information
about the emulsion type. Both dying method and low positive conductivity values
demonstrated the w/o/w type emulsion.
Multiple emulsions yielded low conductivity
values immediately after preparation of formulations. The yield of the
emulsions (entrapment percent, E%) was between 99.6% and 98.7%,
meaning that a portion of the NaCl in the inner aqueous phase, approximately
between 0.4% and 1.3%, leaked into the external aqueous phase during the
preparation of multiple emulsions.
Although the yield values were high,
unfortunately phase separation was observed in some formulations during storage
time at 25 and 40 oC (Table 3).
During storage of w/o/w emulsions, the
conductivity values increased with time (Figure 3). The increase in
conductivity during storage time can be ascribed to the increase in NaCl in the
external aqueous phase due to i) a
diffusion of NaCl through the mineral oil film [35] or ii) the coalescence of internal and external aqueous phases [36], destruction
of oil film because of osmotic pressure and then expulsion of internal aqueous
phase. This most likely explains the
phase separation observed in this study. Due to rising conductivity, declining
viscosity and the approximately stable globule size of multiple emulsions
during storage time, it was concluded that the solute and water molecules were
transported from the oil membrane by a diffusional mechanism.
|
Table 3: Effect of surfactant concentration on
physical stability of multiple emulsions
|
|||||||
|
Abil®EM90 |
Tetronic®908 |
1 month |
2 months |
3 months |
4 months |
5 months |
|
|
2% |
1% |
_ |
_ |
_ |
_ |
no PS
PS at 40o |
|
|
2% |
2% |
_ |
_ |
_ |
no PS at 25o PS at 40o
|
no PS at 25o PS at 40o |
|
|
2% |
4% |
_ |
_ |
_ |
no PS at 25o PS at 40o |
PS at 25o PS at 40o |
|
|
4% |
1% |
_ |
_ |
_ |
_ |
PS at 25o PS at 40o |
|
|
4% |
2% |
_ |
_ |
_ |
PS at 25o PS at 40o |
PS at 25o PS at 40o |
|
|
4% |
4% |
_ |
_ |
PS at 25o PS at 40o |
PS at 25o PS at 40o |
PS at 25o PS at 40o |
|
|
‘―‘
indicates phase separation neither at 25 oC nor at 40 oC;
PS, phase separation |
|||||||
The conductivity profiles showed that the
transport rate of NaCl molecules from inner to outer aqueous phase decreased
with time (first order kinetics) (Figure 3). The cause for the decreasing rate
was interpreted as: The inner aqueous droplets of w/o/w emulsions probably grow
with time due to coalescence of smaller droplets because of the higher
percentage of hydrophilic surfactant. However, in this study, the volume of the
oil phase was constant, and therefore the oil layer covering inner
growing aqueous droplets thickened. Thus, the thickened layer delayed
the transport of electrolyte molecules.
The temperature accelerated the diffusion
or transport of NaCl molecules from inner to external aqueous phase. The
calculated conductivity rate constants (ℓn µS/month) were significantly
different when compared with the formulations having the same composition at 25
or 40 oC (p<0.05). For example, the conductivity rate constants
of multiple emulsions containing 1% Tetronic®908 and 2% Abil®EM90
were calculated as 0.229±0.0183 ℓn µS/month and 0.339±0.03 ℓn
µS/month at 25 and 40 oC, respectively.
The effect of Abil®EM90
concentration on conductivity of the system was investigated, and no
significant difference was obtained among formulations containing 1% Tetronic®908.
Conductivity values were similar with 2 or 4% Abil®EM90
concentration at 25oC. At higher concentrations of Tetronic®908
(2 and 4%), the two-fold increase in lipophilic surfactant did not hinder the
increase in conductivity (Figure 3). Unfortunately, the increase in
concentration of lipophilic surfactant could not provide the physical stability
of the system (Table 3). This finding was in contrast with previous literature
[27, 37, 38].
There was a direct relationship between the
percentage of Tetronic®908 and the conductivity of multiple
emulsions. As could be seen in Figure 3, the conductivity of systems containing
the same amount of lipophilic surfactant increased with increasing Tetronic®908
concentration. This result was also observed by the other investigators [27,
31, 37, 38]. As mentioned previously, the excess
amount of Tetronic®908 in the external aqueous phase probably caused
the formation of micelles and then the solubilizing of Abil®EM90 molecules, thus weakening or rendering unstable
the interface of the w/o system. Therefore, NaCl molecules migrated more easily
from the internal to external aqueous phase.
Viscometric
analysis
Measurement of viscosity gives us useful
data, especially in quality assurance of emulsions. The variation in viscosity
of systems may explain some differences between formulations and at the same
time provide important information about coalescence or globule size change.
The viscosity of the w/o/w multiple
emulsions was measured at four different rpm to follow the time dependence of
viscosity. The viscosity values only at 5 rpm were selected for tables and
figures.
The viscosities of emulsions at first day
after preparation are shown in Table 2.
There are many factors affecting the
viscosity of multiple emulsions; one is the multiple globule size. There was a
direct relationship between the viscosity and globule size of multiple
emulsions. As expected, the minimum
viscosity was observed with the higher globule size (Figure 4). Higher
viscosity may be attributed to the small globule size of multiple emulsions
prepared with the same concentration of Abil®EM90.
Increased hydrophilic surfactant influenced
the viscosity of system, with the viscosity decreasing as the Tetronic®908
concentrations were increased (Table 2), whereas no significant difference in
viscosity as a function of Abil®EM90 percentage was observed
(p>0.05).
All the multiple emulsions exhibited
non-newtonian flow and shear thinning behavior. It means the viscosity of
system decreased with increasing shear stress. The viscosity of multiple
emulsions reduced with increasing shear stress from 5 rpm to 20 rpm. Only one
example of the plots of viscosity versus shear stress (rpm) is shown (Figure
5). In some literatures, the bursting of
multiple globules [39, 40] or phase inversion under shear stress [41] have been
shown, but we did not observe any destruction of multiple globules or phase
inversion after viscosity measurement using optical microscopy. It was
concluded that shear stress used in this study did not induce irreversible
structural changes in multiple emulsions, such as coalescence or phase
inversion.
|
Table 4: Release rate constant of caffeine and some
physical characteristics of multiple emulsions containing caffeine |
||||||||||
|
|
2%
Abil EM90 |
4%
Abil EM90 |
||||||||
|
Tetronic 908, % |
1% |
2% |
4% |
1% |
2% |
4% |
||||
|
kro |
2.31 |
2.89 |
5.66 |
2.56 |
3.82 |
6.95 |
||||
|
(Released %/hour)a |
(0.369)b |
(0.496)b |
(0.511)b |
(0.198)b |
(0.331)b |
(0.552)b |
||||
|
Viscosity (mPa.s) |
54000 |
32000 |
30000 |
56000 |
48000 |
32000 |
||||
|
Globule size (µM) |
25.5 |
34.6 |
36.6 |
19.6 |
20.6 |
35.8 |
||||
|
Released % (±SD)c |
14.8 |
15.9 |
18.3 |
17.2 |
19.0 |
30.9 |
||||
|
(at first two hours) |
(±1.17)c |
(±1.55)c |
(±0.673)c |
(±0.708)c |
(±0.154)c |
(±1.76)c |
||||
|
Released % (±SD) |
26.3 |
26.8 |
41.1 |
26.4 |
27.6 |
54.2 |
||||
|
(at 6th hours) |
(±1.09) |
(±1.09) |
(±1.00) |
(±0.462) |
(±1.51) |
(±0.905) |
||||
|
a r2≥0.997, b SE of regression, c
±standard dev., n=3 |
||||||||||
|
Table 5: Calculated parameters of the kinetic
model suggested by Magdassi and Garti (54) for release of drug from multiple
droplets |
||||||
|
2%
Abil®EM90 |
4% Abil®EM90 |
|||||
|
Tetronic®908 |
1% |
2% |
4% |
1% |
2% |
4% |
|
Slope |
0.00211 |
0.00234 |
0.00714 |
0.00198 |
0.00330 |
0.0123 |
|
Intercept |
0.000205 |
-0.000796 |
-0.00978 |
0.00127 |
-0.00297 |
-0.00839 |
|
r2 |
0.993 |
0.998 |
0.978 |
0.998 |
0.978 |
0.989 |
|
Slope= |
||||||
Viscosities of all the multiple emulsions
decreased continuously during storage with time (Figure 6). This may be due to i) diffusion of water molecules from the
inner to the outer aqueous phase and then the volume decrease of globules in
the w/o/w emulsions, or ii) bursting
of multiple globules due to osmotic pressure. When all multiple emulsions were
compared considering phase separation at 25 oC, systems containing
2% Abil®EM90 and 1 or 2% Tetronic®908 were stable at
least five months, although increasing conductivity and decreasing viscosity
with time were observed. In these formulations, water molecules probably
migrated through the liquid paraffin layer without affecting the entirety of
multiple globules. This has been described by i) micellar transport mechanism, ii) the hydrated surfactant mechanism or iii) diffusion of water molecules across the oil layer between
internal and external aqueous phase [4, 17, 28, 36, 42, 43].
Formation time of multiple globules
The effect of surfactant concentration on
formation time of multiple globules is shown in Figure 7. As can be seen, the
required formation time of multiple emulsions prepared with 2% Abil®EM90
was similar with multiple emulsions prepared with 4% Abil®EM90 for
the same concentration of Tetronic®908. In contrast, the formation
time was significantly affected by the percentage of Tetronic®908; the formation time of
multiple globules increased with an increase in hydrophilic surfactant. It was
considered that Tetronic®908
may influence the interphase between the external aqueous phase and oil layer
(or oil lamella), which has been described by Garti [17]. Abil®EM90
was probably solubilized via the micelles formed by part of the Tetronic
molecules. Thus, as Tetronic concentration increased, formation of multiple
globules was delayed; the higher the Tetronic concentration, the longer the
formation time.
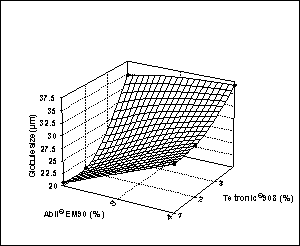
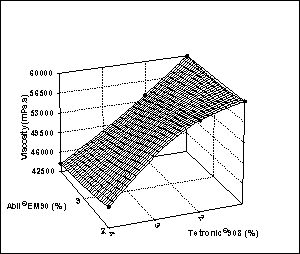
Figure
4: Effect of surfactant
concentration on globule size and viscosity of multiple emulsions.
In
vitro release studies
To investigate the release characteristics
of multiple emulsions, caffeine was used as the model solute. When caffeine was
introduced in the internal aqueous phase of multiple emulsions, the viscosity
and multiple globule size of emulsions changed (Table 4).
The solubility of caffeine was found to be
20.4±0.93 mg/mL (saturation value, Cs) (pH: 5.2). That result agrees
with those of Clément et al. [44] who found that the solubility of caffeine was
19.64±0.59 mg/mL at pH: 7.4.
During release experiments, the release
rate should not be influenced by the concentration gradient between the caffeine
concentration of release medium (Crm) and the saturation
concentration of caffeine (Cs) in release medium. If the Crm
is less than or equal to “Cs x 10/100” for every sampling time, it
is said that a sink condition is maintained. So, when we used 300 ml of release
medium, even if all of the caffeine (0.024 g) in two grams of ME released, Crm
could not be higher than “Cs x 10/100” value (=20.4 x 10/100= 2.04
mg/ml). Therefore, it was concluded that the volume of dialysis medium was not
rate limiting in diffusion of caffeine and the release experiments were
conducted under sink conditions.
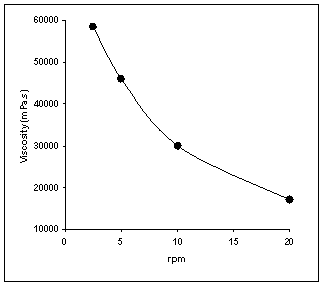
Figure
5: Viscosity as a function
of shear stress – Non-Newtonian flow behavior of multiple emulsion
containing 2% Abil®EM90 and 2% Tetronic®908
The cumulative percent release of caffeine
from multiple emulsions is shown in Figures 8 and 9. Points and bars in each
figure represent mean value ±standard error (SE). When the SE was smaller than
the size of symbol, no bar was shown.
All release profiles exhibited an initial
rapid release phase and then a slower release of molecules. First phase of
release profiles (the first hour of profile) might be due to caffeine existence
in the external aqueous phase during the manufacturing; during the second
emulsification, caffeine molecules probably leaked out of the internal aqueous
phase. Therefore, the secondary emulsification was stopped immediately after
observation of multiple globules on microscope.
The drug molecules at the external aqueous phase were free molecules
that were released immediately. The release of caffeine in the external phase
of w/o/w emulsions was completed within approximately 2 hours. Negligible drug
release from multiple emulsions was observed between the 1st and 2nd
hours. This may be attributed to the slow transport of caffeine from the
internal aqueous phase; a period of time was probably required for caffeine
molecules to transport from internal droplets to the dialysis membrane.
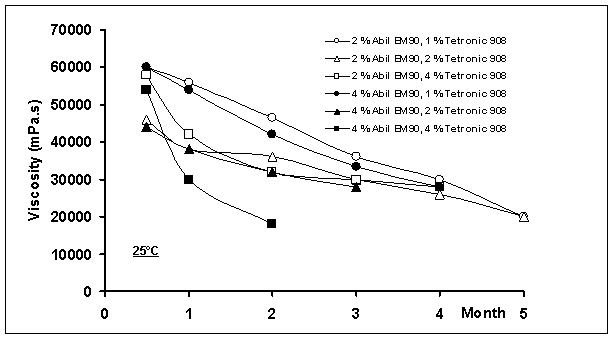
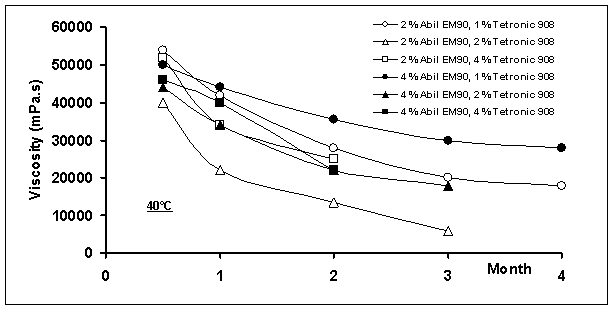
Figure
6: The decreasing of viscosity
as a function of time at 25 and 40oC (shear stress: 5 rpm)
After 2 hours, caffeine in the internal
aqueous phase was released slowly at a rate governed by the interphase barrier
between the inner and outer aqueous phases because the hydrophilic drug
molecules could not pass freely through the oily layer. So the second phase
might be attributed to a prolonged release of caffeine from the internal
aqueous phase.
The release rates presented in Table 4 were
determined from slopes of the release percentage versus time between the 2nd
and 6th hours. These rates were used the explanation and comparison
of the effect of surfactant concentration on the release profiles.
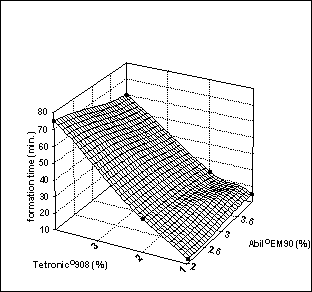
Figure
7: Effect of surfactant
concentration on formation time of multiple globules.
The
effect of hydrophilic surfactant concentration
Profiles shown in Figures 8 and 9 indicate
that emulsions containing 4% Tetronic®908 enhanced the drug released
in comparison with the other concentration of this surfactant. It was also
observed that multiple emulsions containing 1 or 2% of Tetronic®908
gave almost similar patterns of caffeine levels. When the release percentages at the end of
the release experiment were compared, the difference between values was not
very pronounced, except for the emulsions containing 4% Tetronic®908. In fact, the release percentages of caffeine
from formulations containing 1 or 2% Tetronic®908 after 6 hours were not significantly different
(p=0.849). On the contrary, when the release percentages for multiple emulsions
prepared with 4% Tetronic®908
were compared after 6 hours, the difference was statistically significant
(p=0.0297), 41.1 and 54.2% of the total dose. When we used the ANOVA, it was
found that there was the significant difference between the release rates of
MEs containing different amount of hydrophilic surfactant (p=0.017).
Although the release rate or release % of
caffeine for formulation containing 4% Tetronic®908 was relatively large, the physical stability of
those emulsions was not better than the other MEs containing 1% or 2% Tetronic®908 as shown in Table 3;
the high concentration of hydrophilic surfactant did not improve the physical
stability of system. This finding agrees with those of Matsumoto et al. [27]
and Jiao and Burgess [31]. These authors suggested that the usage of a high
concentration of hydrophilic surfactant caused the solubilization of lipophilic
surfactant and then disruption of multiple globules. In our study, the higher
concentration of Tetronic®908 in the outer aqueous phase may have
served to solubilize the molecules of the lipophilic surfactant, which probably
led to the rupture of the oil layer and then the rapid release of molecules of
caffeine to the external aqueous phase. In addition, this situation probably
caused the phase separation.
In this study, Tetronic®908 was
used in concentrations of 1, 2 and 4%, and these percentages are much higher
than critical micelle concentration defined by Atwood et al. [45] and Dong et
al. [46], 0.020% at 40 oC and 0.16% at 25 oC,
respectively. Therefore, Tetronic®908 chains probably formed
micelles in both aqueous and oil phases and this facilitated the transport of
caffeine molecules through the oil phase, as described Omotosho et al [47].
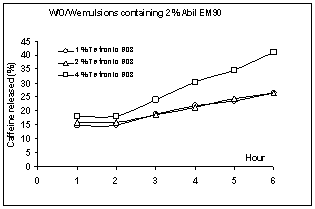
Figure
8: Release profiles of
caffeine from w/o/w multiple emulsions containing different concentration of
Tetronic®908 (mean±SE, n: 3)
The
effect of lipophilic surfactant concentration
Previous literatures have shown that the
percentage of the lipophilic surfactant plays an important role in the release
amount of active molecule. In some of these reports, when the lipophilic
surfactant concentration was increased, the release of solute molecules
decreased due to maximum viscosity of the system. The cause of increasing
viscosity has been explained as the maximum swelling of multiple globules of
the system [37, 48-51]. In contrast, in other reports, an increase in
lipophilic surfactant amount caused an increase in the release rate of solute
and water [17, 47, 52, 53]. This has been explained by
the authors as follows: During the first emulsification, the reverse micelles
form in the oil phase of w/o primary emulsion due to the higher concentration
of lipophilic surfactant that is above critical value. In addition, during the
second emulsification, the existence of hydrophilic surfactant may cause the
formation of mixed inverse micelles. Thus, both water and water-soluble
molecules in the micelles could be easily carried across the oil layer between
the internal and external aqueous phase of w/o/w emulsion.
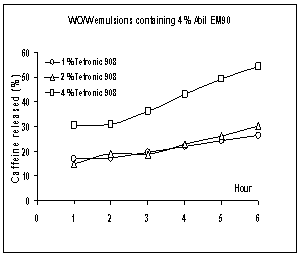
Figure
9: Release profiles of
caffeine from w/o/w multiple emulsions containing different concentration of
Tetronic®908 (mean±SE, n: 3).
In this study, the effect of Abil®EM90
concentration on the release rate or release amount of caffeine is shown in
Table 4. It can be seen that as the concentration of lipophilic surfactant in
the primary emulsion (w/o) increased, the release rate of caffeine from
multiple emulsions increased. In
addition, the percentage of caffeine released from multiple emulsions
containing 4% Abil®EM90
was greater than that of the other multiple emulsions containing 2% Abil®EM90 prepared with same
concentration of hydrophilic surfactant. The release rates of MEs containing 2%
of lipophilic surfactant were compared to the release rates of MEs containing
4% of lipophilic surfactant using ANOVA test. It was found that the difference
was not statistically significant (p=0.114).
In our study, the droplet size of w/o
primary emulsion was approximately the same for two concentrations of Abil®EM90, which likely indicates
that the two-fold increase of lipophilic surfactant no longer significantly
decreased the interfacial tension. Thus, it is plausible to assume that a
concentration of 2% Abil®EM90
was just sufficient to cover the inner aqueous phase of the primary emulsion;
the excess amount was likely located in a molecular and micellar form
(containing water, caffeine and NaCl) in the oily phase of the primary
emulsion. So, when a higher concentration of Abil®EM90 is used in multiple emulsions, caffeine molecules
in the inner aqueous phase may be transported by micelles in addition to the
molecular diffusion.
The release studies showed that the release
rate of caffeine was faster for those systems with lower viscosity at the same
percentage of Abil®EM90;
this also depended on increasing Tetronic®908
concentrations. The viscosity of systems was more effectual than the globule
size of multiple emulsions, because emulsions with smaller globules were unable
to generate the faster release rate. Therefore, although the globule size did
not affect the rate of drug released, the viscosity of multiple emulsions did
due to consistency of the system.
Based on results of release experiments, it
was concluded that caffeine might be transported out by molecular diffusion and
through a reverse micellar mechanism controlled by the viscosity of the
system.
The
possible kinetic model for release of caffeine from multiple droplets
In the previous studies, Higuchi’s
mechanism for release of molecule from dispersed polymeric matrix was modified
and adapted for MEs [16, 53, 54]. Magdassi and Garti
applied this model to w/o/w type of MEs and defined the following equation
[54]:
![]()
where F is the fraction of the drug release from
globule; D is the diffusion coefficient of drug through oil membrane; Cs is the drug solubility in
the oil membrane between the inner aqueous phase and the outer aqueous phase; ro is the radius of the mean
globule size of ME; Co is
the initial concentration of the drug. For simplification the value of “![]() “ was called B.
“ was called B.
In our study, we used this equation and the
calculated B values were plotted
against time, t (Figure 10). The
caffeine solubility in the paraffin film and “D” value were assumed to be
constant. The swelling of the globules of MEs was also cancelled, because as
mentioned before the mean size of multiple globules was not changed during time
(Figure 2).
As shown in Figure 10, the profiles
exhibited two phase for all the MEs. This was the unexpected situation; the
relationship between B and t could be the linear. Sela et al [53]
explained the first phase of profile as resulting from the time required for
the formation of reverse micelles and solubilization of water and drug in these
micelles. The second and linear phase was the straight line as expected; this
confirmed the validity of the equation and the r2 values was higher
than 0.978 (Table 5). As a result, it
can be concluded that the release of the caffeine molecules through the
paraffin film probably was controlled by diffusion mechanism.
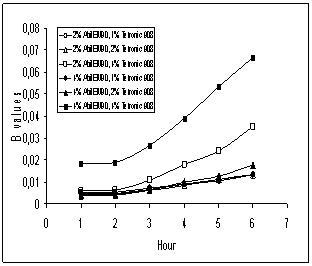
Figure
10: B versus t profiles for
release of caffeine from multiple droplets
CONCLUSIONS
The investigations presented lead us to
conclude that the multiple emulsions prepared with Tetronic®908 were highly uniform and high yields of w/o/w
multiple emulsions were obtained. Relatively stable systems were formed using
Tetronic®908 and Abil®EM90 surfactant pair. It is
possible to formulate an optimal multiple emulsions consisting of Tetronic®908 (1%) in the outer
aqueous phase and Abil®EM90
(2%) in the oil phase. The results
indicated that Tetronic®908
as hydrophilic surfactant might be good emulsifier for the preparation of w/o/w
type emulsions. Stability measurements showed that the concentration of
hydrophilic and lipophilic surfactant were very important parameter. The
release of caffeine as model hydrophilic substance from system studied; an
initial rapid release followed by a much slower rate of release was obtained.
Multiple w/o/w emulsion systems can be utilized as potential prolonged release
dosage forms. It was concluded that surfactant concentrations affected the
release rate.
Further in-vitro examinations such as
different type of oil phase or the different combinations of aqueous phase are
still required and it is recognized that further formulation studies are needed
to obtain the most stable formulations using Tetronic®908.
REFERENCES
1)
De Luca, M., Vaution, C.,
Rabarron, A., Seiller, M., Classification et obtention des emulsions multiples.
S.T.P. Pharma. Sci., 4(8):
679-687, 1988.
2)
De Luca, M., Grossiord, J.L., Médard, J.M., Vaution, C., A stable w/o/w
multiple emulsion.Cos. Toilet., 105: 65-69, 1990.
3)
Taelman,
M.C., Loll, P., Multiple emulsions in cosmetics. ICI Surfactants, Reprint,
RP112/94E, 1994.
4)
Garti, N., Aserin, A., Double emulsions
stabilized by macromolecular surfactants. Adv. Coll. Interface. Sci., 65:
37-69, 1996.
5)
Omotosho, J.A.,
6)
Ferreira, L.A.M., Doucet, J., Seiller, M.,
Grossiord, J.L., Marty, J.P., Wepierre, J., In vitro percutaneous absorption of
metronidazole and glucose: Comparison of o/w, w/o/w and w/o systems. Int. J.
Pharm., 121: 169-179, 1995.
7)
Silva-Cunha, A., Chéron, M., Grossiord,
J.L., Puisieux, F., Seiller, M., W/O/W multiple emulsions of insulin containing
a protease inhibitor and an absorption enhancer: Biological activity after oral
administration to normal and diabetic rats. Int. J. Pharm., 169: 33-44, 1998.
8)
Silva-Cunha, A., Grossiord, J.L., Seiller,
M., Multiple Emulsions: Pharmaceutical Potentiality, in: Grossiord JL: Seiller M (eds.), Multiple Emulsions:
Structure, Properties and Applications, Editions
9)
Jager-Lezer, N., Denine, R., Grossiord,
J.L., Wepierre, J., Rault, S., Seiller, M., Formulating multiple emulsions with
moisturizing actives. Cosm. Toilet., 111: 53-58, 1996.
10)
Omotosho, J.A., Whateley, T.L.,
11)
Okochi, H., Nakano, M., Basic studies on
formulation, method of preparation and characterization of
water-in-oil-in-water type multiple emulsions containing vancomycine. Chem.
Pharm. Bull., 44: 180-186, 1996.
12)
Doucet, O., Ferrero, L., Garcia, N., Zastrow, L., O/W emulsion
and w/o/w multiple emulsions: Physical characterization and skin
pharmacokinetic comparison in the delivery process of caffeine. Int. J. Cosm.
Sci., 20: 283-295, 1998.
13)
Okochi, H., Nakano, M., Preparation and
evaluation of w/o/w type emulsions containing vancomycin. Adv. Drug. Del. Rev.,
45: 5-26, 2000.
14)
Morishita, M., Matsuzawa, A., Takayama, K.,
Isowa, K., Nagai, T., Improving insulin enteral absorption using
water-in-oil-in-water emulsion. Int. J. Pharm., 172: 189-198, 1998.
15)
Gallarate, M.,
16)
Raynal, S., Grossiord, J.L., Seiller, M.,
Clausse, D., A topical w/o/w multiple emulsion containing several active
substances: formulation, characterization and study of release.J. Cont. Rel.,
26: 129-140, 1993.
17)
Garti, N., Double emulsions – Scope,
limitations and new achievements. Coll.
Surf. A: Physicochem. Engineer. Asp., 123-124:
233-246, 1997.
18)
Seiller, M., Grossiord, J.L., Silva-Cunha,
A., Multiple Emulsions: Pharmaceutical Potentiality, in: Grossiord JL: Seiller M (eds.), Multiple Emulsions:
Structure, Properties and Applications, Editions
19)
Schmolka, I.R., )
A comparison of block copolymer surfactant gels. J. Am. Oil. Chem. Soc.
(JAOCS), 68: 206-209, 1991.
20)
Clément, P., Laugel, C., Marty, J.P., In
vitro release of caffeine from concentrated w/o emulsions: Effect of
formulation parameters. Int. J. Pharm., 207: 7-20, 2000.
21)
Olivieri, L., Seiller, M., Bromberg, L.,
Besnard, M., Duong, T.N.L., Grossiord, J.L., Optimization of a thermally reversible
w/o/w multiple emulsion for shear-induced drug release. J. Cont. Rel., 88:
401-412, 2003.
22)
Ollivon, M., Potier-Guméry, L., Multiple
Emulsions: Pharmaceutical Potentiality, in: Grossiord JL: Seiller M (eds.), Multiple Emulsions:
Structure, Properties and Applications, Editions
23)
Aronson, M.P., Petko, M.F.,
Highly concentrated water in oil emulsions: Influence of electrolyte on their
properties and stability. J. Colloid Interface Sci., 159: 134-149, 1993.
24)
Kawashima, Y., Hino, T., Takeuchi, H.,
Niwa, T., Stabilization of water/oil/water multiple emulsion with hypertonic
inner aqueous phase. Chem. Pharm. Bull.
40: 1240-1246, 1992.
25)
Opawale, F.O., Burgess, D.J., Influence of
interfacial properties of lipophilic surfactants on water-in-oil emulsion
stability. J. Colloid. Interface. Sci., 197: 142-150, 1998.
26)
Kanouni, M., Rosano, H.L., Naouli, N., Preparation of a stable double emulsion (w1/o/w2):
Role of the interfacial films on the stability of the system. Adv. Coll. Interface. Sci., 99: 229-254,
2002.
27)
Matsumoto, S., Kıta, Y., Yonezawa, D.
J., An attempt at preparing water-in-oil-water multiple-phase emulsions. Coll.
Interface Sci., 57: 353-361, 1976.
28)
Magdassi, S., Frenkel, M., Garti, N., On the factors affecting the yield of preparation and
stability of multiple emulsions.J. Dispers. Sci. Tech., 5: 49-59, 1984.
29)
Laugel, C., Chaminade, P., Baillet, A.,
Seiller, M., Ferrier, D., Moisturizing substances entrapped in w/o/w emulsions:
Analytical methodology for formulation, stability and release studies. J. Cont.
Rel., 38: 59-67, 1996.
30)
Opawale, F.O., Burgess, D.J., Influence of
interfacial rheological properties of mixed emulsifier films on the stability
of water-in-oil-in-water emulsions. J. Pharm. Pharmacol., 50: 965-973, 1998.
31)
Jiao, J., Burgess, D.J., AAPS PharmSci., Rheology
and stability of water-in-oil-in-water multiple emulsions containing Span 83
and Tween 80. 5: Article 7, 2003 (http://www.pharmsci.org).
32)
33)
Omotosho, J.A., The effect of acacia,
gelatin and polyvinylpyrrolidone on chloroquine transport from multiple w/o/w
emulsions. Int. J. Pharm., 62: 81-84,
1990.
34)
Sever, S., Ocak (Tırnaksız), F.,
A topical w/o/w multiple emulsion prepared with Pluronic F-127 or Tetronic 908
as a hydrophilic surfactant: Formulation, characterization and release Studies,
in Yazan Y: Baser KHC (eds.), 4th
International Cosmetics Symposium, 7-8 June 2000, Istanbul, pp 212, 2000.
35)
Pays, K., Giermanska-Kahn, J., Pouligny,
B., Bibette, J., Leal-Calderon, F., Double emulsions: How does release occur?.J. Cont. Rel., 79: 193-200, 2002.
36)
Wen, L., Papadopoulos, K.D., Osmotic
pressure on water transport in w1/o/w2 emulsions. J.
Coll. Interface Sci., 235: 398-404, 2001.
37)
Jager-Lezer, N., Terrisse,
38)
Csóka, I., Erős,
39)
Terrisse,
40)
Muguet, V., Seiller, M., Barratt, G., Ozer,
O., Marty, J.P., Grossiord, J.L., Formulation of shear rate sensitive multiple
emulsions J. Cont. Rel., 70: 37-49, 2001.
41)
Kawashima, Y., Hino, T., Takeuchi, H.,
Niwa, T., Horibe, K., Rheological study of w/o/w emulsion by a cone-and-plate
viscometer: Negative thixotropy and shear-induced phase inversion. Int. J.
Pharm., 72: 65-77, 1991.
42)
Magdassi, S., Garti, N., Release of
electrolytes in multiple emulsions: Coalescence and breakdown or diffusion
through oil phase?.
Coll. Surf., 12: 367-373, 1984.
43)
Wen, L., Papadopoulos, K.D., Visualization
of Water Transport in W1/O/W2 Emulsions.Coll. Surf. A:Physicochem
44)
Clément, P., Laugel, C., Marty, J.P.,
Influence of three synthetic membranes on the release of caffeine from
concentrated w/o emulsions. J. Cont. Rel., 66: 243-254, 2000.
45)
Attwood, D., Collett, J.H., O’Connor,
46)
Dong, J., Chowdhry, B.Z., Leharne, S.A.,
Surface activity of poloxamines at the interfaces between air-water and
hexane-water. Coll. Surf. A: Physicochem
47)
Omotosho, J.A., Whateley, T.L.,
48)
Geiger, S., Tokgoz, S., Fructus, A.,
Jager-Lezer, N., Seillere, M., Lacombe, C., Grossiord, J.L., Kinetics
of swelling-breakdown of a w/o/w multiple emulsion: Possible mechanisms for the
lipophilic surfactant effect. J. Cont. Rel., 52: 99-107, 1998.
49)
Geiger, S., Jager-Lezer, N., Tokgoz, S.,
Seiller, M., Grossiord, J.L., Characterization of the mechanical properties of
a water/oil/water multiple emulsion oily membrane a micropipette aspiration
technique.Coll. Surf. A: Physicochem. Engineer. Asp.,
157: 325-332, 1999.
50)
Yan, J., Pal, R.J., W/O/W multiple
emulsions: A review of release mechanisms by break-up of the oily membrane. J.
Memb. Sci., 190: 79-91, 2001.
51)
Grossiord, J.L., Seiller, M., W/O/W
multiple emulsions: A review of release mechanisms by break-up of the oily
membrane. STP Pharma. Sci., 11: 331-339, 2001.
52)
Omotosho, J.A., Whateley, T.L., Law, T.K.,
53)
Sela, Y., Magdassi, S.,
54)
Magdassi, S., Garti, N., A kinetic model
for release of electrolytes from w/o/w multiple emulsions. J. Cont. Rel., 3:
273-277, 1986.
Published by the Canadian Society for Pharmaceutical Sciences.
Copyright © 1998 by the Canadian
Society for Pharmaceutical Sciences.
http://www.cspscanada.org
CSPS Home | JPPS
Home | Search
| Subscribe to JPPS