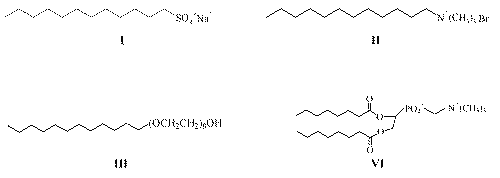J Pharm Pharmaceut Sci (www.cspscanada.org) 8(2):147-163, 2005
Micellar solubilization of drugs.
Carlota Oliveira Rangel-Yagui1, Adalberto Pessoa Junior, Leoberto Costa Tavares
Department of Biochemical and Pharmaceutical Technology /FCF, University of São Paulo, São Paulo, BrazilReceived 17 November 2004, Revised 2 February 2005, Accepted 4 March 2005, Published 8 July 2005
Abstract
PURPOSE: Micellar solubilization is a powerful alternative for dissolving hydrophobic drugs in aqueous environments. In this work, we provide an insight into this subject. METHODS: A concise review of surfactants and micelles applications in pharmacy was carried out. RESULTS: Initially, a description of surfactants and aqueous micellar systems is presented. Following, an extensive review on micellar drug solubilization, including both the principles involved on this phenomenon and the work already done regarding solubilization of drugs by micelles is presented. The application of micelles in drug delivery, in order to minimize drug degradation and loss, to prevent harmful side effects, and to increase drug bioavailability, is also presented. Special emphasis is given to the more recent use of polymeric micelles. Finally, we briefly discuss the importance of surfactants and micelles as biological systems models as well as its application in micellar catalysis. CONCLUSIONS: As can be seen from the review presented, the use of micelles in pharmacy is an important tool that finds numerous applications.
Introduction
Surfactants are known to play a vital role in many processes of interest in both fundamental and applied science. One important property of surfactants is the formation of colloidal-sized clusters in solutions, known as micelles, which have particular significance in pharmacy because of their ability to increase the solubility of sparingly soluble substances in water (1). Micelles are known to have an anisotropic water distribution within their structure. In other words, the water concentration decreases from the surface towards the core of the micelle, with a completely hydrophobic (water-excluded) core. Consequently, the spatial position of a solubilized drug in a micelle will depend on its polarity: nonpolar molecules will be solubilized in the micellar core, and substances with intermediate polarity will be distributed along the surfactant molecules in certain intermediate positions.
On the other hand, numerous drug delivery and drug targeting systems have been studied in an attempt to minimize drug degradation and loss, to prevent harmful side effects, and to increase drug bioavailability (2-6). Within this context, the utilization of micelles as drug carriers presents some advantages when compared to other alternatives such as soluble polymers and liposomes. Micellar systems can solubilize poorly soluble drugs and thus increase their bioavailability, they can stay in the body (blood) long enough to provide gradual accumulation in the required area, and their sizes permit them to accumulate in areas with leaky vasculature (7).
In general, surfactants play an important role in contemporary pharmaceutical biotechnology, since they are largely utilized in various drug dosage forms to control wetting, stability, bioavailability, among other properties (8). It is important to notice that lyophobic colloids, such as polymers, require certain energy to be applied for their formation, are quite unstable from the thermodynamic point of view, and frequently form large aggregates. Association colloids such as micelles, on the other hand, can form spontaneously under certain conditions (self-assembling systems), and are thermodynamically more stable towards both dissociation and aggregation (9).
Therefore, the study of surfactants and their role in pharmacy is of paramount importance, especially with respect to their ability of solubilizing hydrophobic drugs. In this work, we provide a review of micellar solubilization of drugs in surfactant systems, blending it with basic information on surfactants structure and properties, as well as the applications for drug delivery.
Surfactants and Micelles
Surfactants are amphiphilic molecules composed of a hydrophilic or polar moiety known as head and a hydrophobic or nonpolar moiety known as tail. The surfactant head can be charged (anionic or cationic), dipolar (zwitterionic), or non-charged (nonionic). Sodium dodecyl sulfate (SDS), dodecyltrimethylammonium bromide (DTAB), n -dodecyl tetra (ethylene oxide) (C 12 E 4 ) and dioctanoyl phosphatidylcholine (C 8 -lecithin) are typical examples of anionic, cationic, nonionic and zwitterionic surfactants, respectively (Figure 1). The surfactant tail is usually a long chain hydrocarbon residue and less often a halogenated or oxygenated hydrocarbon or siloxane chain (16, 17).
Figure 1: Examples of I-anionic (SDS), II-cationic (CTAB), III- nonionic (C12 E4) and VI-zwitterionic (C8 -lecithin) surfactants.
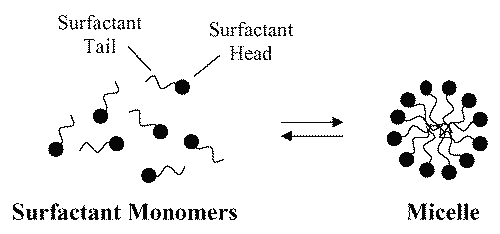
A surfactant, when present at low concentrations in a system, adsorbs onto surfaces or interfaces significantly changing the surface or interfacial free energy. Surfactants usually act to reduce the interfacial free energy, although there are occasions when they are used to increase it (17). When surfactant molecules are dissolved in water at concentrations above the critical micelle concentration (cmc) , they form aggregates known as micelles. In a micelle, the hydrophobic tails flock to the interior in order to minimize their contact with water, and the hydrophilic heads remain on the outer surface in order to maximize their contact with water (see Figure 2) (18,19). The micellization process in water results from a delicate balance of intermolecular forces, including hydrophobic, steric, electrostatic, hydrogen bonding, and van der Waals interactions. The main attractive force results from the hydrophobic effect associated with the nonpolar surfactant tails, and the main opposing repulsive force results from steric interactions and electrostatic interactions between the surfactant polar heads. Whether micellization occurs and, if so, at what concentration of monomeric surfactant, depends on the balance of the forces promoting micellization and those opposing it (19, 20).
Figure 2: Schematic illustration of the reversible monomer-micelle thermodynamic equilibrium. The black circles represent the surfactant heads (hydrophilic moieties) and the black curved lines represent the surfactant tails (hydrophobic moieties).
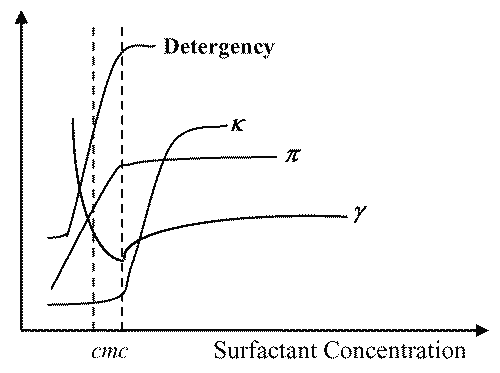
The determination of a surfactant cmc can be made by use of several physical properties, such as surface tension (y), conductivity (k) - in case of ionic surfactants, osmotic pressure (p), detergency, etc. When these properties are plotted as a function of surfactant concentration (or its logarithm, in case of surface tension), a sharp break can be observed in the curves obtained evidencing the formation of micelles at that point (16) (Figure 3).
Figure 3: Changes in the physical properties detergency, conductivity (k), osmotic pressure (p) and surface tension (y) of an aqueous solution of surfactant as a function of surfactant concentration. The break in the curve of each property corresponds to the Critical Micelle Concentration (cmc).
Another important parameter that characterizes micelles is the aggregation number, N ag , that corresponds to the average number of surfactant monomers in each micelle of a micellar solution. Usually, in a micellar solution the aggregation number is approximately constant for a broad total concentration range (up to about 100 times the cmc), with the number of micelles varying (21). However, at certain conditions micelles can grow with the aggregation number varying with the surfactant concentration. (22).
Micelles are labile entities formed by the noncovalent aggregation of individual surfactant monomers. Therefore, they can be spherical, cylindrical, or planar (discs or bilayers). Micelle shape and size can be controlled by changing the surfactant chemical structure as well as by varying solution conditions such as temperature, overall surfactant concentration, surfactant composition (in the case of mixed surfactant systems), ionic strength and pH. In particular, depending on the surfactant type and on the solution conditions, spherical micelles can grow one-dimensionally into cylindrical micelles or two-dimensionally into bilayers or discoidal micelles. Micelle growth is controlled primarily by the surfactant heads, since both one-dimensional and two-dimensional growth require bringing the surfactant heads closer to each other in order to reduce the available area per surfactant molecule at the micelle surface, and hence the curvature of the micelle surface (18, 22).
For all these micellar structures in aqueous media, the surfactant molecules are oriented with their polar heads towards the water phase and their tail away from it. In ionic micelles, the interfacial region between the micelle and the aqueous phase contains the ionic head groups, the Stern Layer of the electrical double layer related to these groups, approximately half of the counter ions associated with the micelle, and water. The remaining counter ions are contained in the Gouy-Chapman portion of the double layer that extends further into the aqueous phase. The length of the double layer is a function of the ionic strength of the solution and it can be highly compressed in the presence of electrolytes (23). For the nonionic surfactants having a polyethylene oxide (PEO) head group, the structure is essentially the same, except that the counter ions are not present in the outer region, but rather coils of hydrated polyethylene oxide chains. The interior of the micelle containing the hydrophobic groups presents a radius of approximately the length of the fully extended hydrophobic chain (17). Another important characteristic of micelles is that the aqueous phase penetrates into the micelle beyond the hydrophilic head groups, and the first few methylene groups adjacent to the head are considered in the hydration sphere. Therefore, we can divide the interior region of the micelle in an outer core penetrated by water and in an inner core completely water-excluded (22).
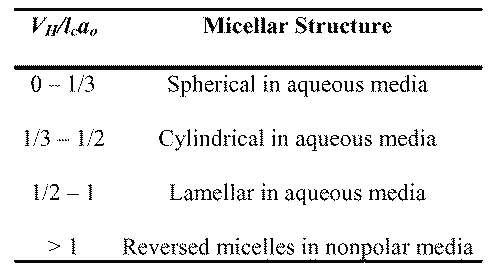
Based on the geometry of various micellar shapes and the space occupied by the hydrophilic and hydrophobic groups of the surfactants, it is possible to estimate the structure of a micelle (20). Accordingly, the parameter VH/lcao can determine the shape of the micelle, with VH corresponding to the volume of the hydrophobic group in the micellar core, lc is the length of the hydrophobic group in the core and ao the cross-sectional area occupied by the hydrophilic group at the micelle-solution interface. Based on Tanford (19), VH = 27.4 + 26.9 n Å, where n is the number of carbon atoms in the chain less one, and lc = 1.5 + 1.265 n Å, depending upon the extension of the chain. Therefore, for a fully extended chain, lc = 1.5 + 1.265 n Å (Table 1).
Table 1: Correlation between the parameter VH/lcao and the structure of the micelle.
Micellar Solubilization
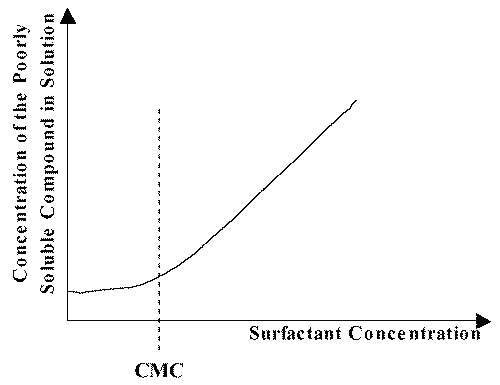
An important property of micelles that has particular significance in pharmacy is their ability to increase the solubility of sparingly soluble substances in water. In this context, solubilization can be defined as the spontaneous dissolving of a substance by reversible interaction with the micelles of a surfactant in water to form a thermodynamically stable isotropic solution with reduced thermodynamic activity of the solubilized material (17). If we plot the solubility of a poorly soluble compound as a function of the concentration of surfactant, as shown in Figure 4, usually what happens is that the solubility is very low until the surfactant concentration reaches the cmc. At surfactant concentrations above the cmc the solubility increases linearly with the concentration of surfactant, indicating that solubilization is related to micellization.
Figure 4: Schematic plot of the concentration of a poorly soluble compound as a function of the surfactant concentration in aqueous solution.
From the thermodynamic point of view, the solubilization can be considered as a normal partitioning of the drug between two phases, micelle and aqueous, and the standard free energy of solubilization ( ΔGSº ) can be represented by the following expression (1):
(1)
where R is the universal constant of the gases, T is the absolute temperature, and P is the partition coefficient between the micelle and the aqueous phase.
Usually, the solubilization of a molecule by a surfactant can be evaluated based on two descriptors that are the molar solubilization capacity, χ , and the micelle-water partition coefficient, P (24). The χ value is defined as the number of moles of the solute (drug) that can be solubilized by one mol of micellar surfactant, and characterizes the ability of the surfactant to solubilize the drug. It can be calculated based on the general equation for micellar solubilization:
(2)
where S tot is the total drug solubility, S W is the water drug solubility, C surf is the molar concentration of surfactant in solution, and cmc is the critical micelle concentration (25). Since above the cmc the surfactant monomer concentration is approximately equal to the cmc , the term (C surf - cmc ) is approximately equal to the surfactant concentration in the micellar form and, therefore, χ is equal to the ratio of drug concentration in the micelles to the surfactant concentration in the micellar form.
On the other hand, the micelle-water partition coefficient is the ratio of drug concentration in the micelle to the drug concentration in water for a particular surfactant concentration, as follows:
(3)
Combining Equations (2) and (3), we can relate the two solubility descriptors. Accordingly, for a given surfactant concentration:
(4)
As can be seen, P is related to the water solubility of the compound, in contrary to χ (25). In order to eliminate the dependence of P on the surfactant concentration, a molar micelle-water partition coefficient (PM), corresponding to the partition coefficient when Csurf = 1 M, can be defined as follows:
(5)
The lower is the cmc value of a given surfactant, the more stable are the micelles. This is especially important from the pharmacological point of view, since upon dilution with a large volume of the blood, considering intravenous administration, only micelles of surfactants with low cmc value still exist, while micelles from surfactants with high cmc value may dissociate into monomers and their content may precipitate in the blood (26).
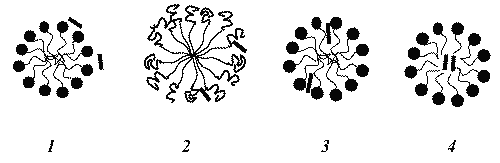
There are a number of possible loci of solubilization for a drug in a micelle, as represented in Figure 5.
Figure 5: Possible loci of solubilization of drugs in surfactant micelles, depending on the drug hydrophobicity. The black bold lines (—) represent the drug at different sites in the micelle. The black circles represent the surfactant heads, the black bold curved lines represent surfactant heads consisting of PEO, and the light black curved lines represent the surfactant tails.
Accordingly, hydrophilic drugs can be adsorbed on the surface of the micelle (1), drugs with intermediate solubility should be located in intermediate positions within the micelle such as between the hydrophilic head groups of PEO micelles (2) and in the palisade layer between the hydrophilic groups and the first few carbon atoms of the hydrophobic group, that is the outer core (3), and completely insoluble hydrophobic drugs may be located in the inner core of the micelle (4) (7,17). The existence of different sites of solubilization in the micelle results from the fact that the physical properties, such as microviscosity, polarity and hydration degree, are not uniform along the micelle (27).
Mukerjee and Cardinal (28) studied the microenvironments of benzene, some of its derivatives, Triton X-100, and naphthalene when solubilized in micelles at low solubilizate to surfactant ratios and proposed the existence of at least two states ( loci ) of solubilization with different polarity. According to the authors, the total uptake by micelles could be divided approximately into an "adsorbed" fraction (location at the micelle-water interface) and a "dissolved" fraction (location in the hydrocarbon core). When adsorption takes place the solubility increases beyond the solubility power of the hydrocarbon core. In fact, numerous studies indicate that the solubility of slightly polar substances and aromatic compounds tend to be considerably higher than the solubility of aliphatic compounds presenting similar molar volumes, despite the fact that the later are expected to be more compatible with the aliphatic hydrocarbon core of most micelles (28).
The capacity of surfactants in solubilizing drugs depends on numerous factors, such as chemical structure of the surfactant, chemical structure of the drug, temperature, pH, ionic strength, etc (7). Nonionic surfactants usually are better solubilizing agents than ionic surfactants for hydrophobic drugs, because of their lower cmc values. For polar drugs it is more complicate to establish a general relationship between the degree of solubilization and the chemical structure of the surfactant, since solubilization can be in both the inner and the outer regions of the micelle. Krishna and Flanagan (29) observed that, for the antimalarial drug b -Arteether (an endoperoxide containing a sesquiterpene lactone), nonionic surfactants showed much lower solubilization power than ionic surfactants. They suggested that the solubilization of this drug may not only involve incorporation into the micellar interior, but may be substantially due to adsorption at the micelle-water interface.
Regarding the influence of structure of the drug, crystalline solids generally show less solubility in micelles than do liquids of similar structure (17). For polar drugs, the depth of penetration into the micelle varies with the structure of the drug. Usually, the less polar the drug (or the weaker its interaction with either the polar head of the surfactant in the micelle or the water molecules at the micelle-water interface) and the longer is the chain length, the smaller its degree of solubilization, reflecting its deeper penetration into the palisade layer (17,23).
The extent of solubilization into a particular micelle depends upon the locus of solubilization and therefore the shape of the micelle. As described previously, the shape of the micelle is determined by the value of the parameter VH/lcao and as this parameter increases the micelle becomes more asymmetrical and the volume of the inner core increases relative to that of the outer portion. Therefore, one can expect that the solubilization of drugs in the core will increase with increase in asymmetry, whereas the solubilization of drugs in the outer region will decrease (17). In fact, it was observed that for alkyl sodium sulfates and alkyl trimethylammonium bromides, the solubilization of b-Arteether increases with the increase in the alkyl chain length, due to the larger micellar size (29).
However, Barry and El Eini (30) studying the solubilization of non-polar steroidal drugs in aqueous solutions of long-chain polyoxyethylene nonionic surfactants have observed that the molar solubilizing efficiency of surfactants increased as the length of the PEO chain increased while micellar sizes are known to decrease with the increase in PEO chain length. The authors suggested that, although the inclusion of non-polar steroids into the micelles decreases as the PEO hydrophilic chain increases, the number of micelles in equimolar amounts of surfactants increases and consequently the total amount of steroid per mole of surfactant is greater, hence the observed increase in solubilizing efficiency with increased hydrophilic chain length when molar concentrations are considered.
Ong and Manoukian (31) have studied the solubilization of timobesone acetate, a corticosteroid used in inflammatory therapy, in nonionic surfactants solutions and observed that the solubilization capacity increased with increasing length of the hydrophobic tail of the surfactants. Therefore, timobesone was assumed to be solubilized in the hydrophobic core of the micelles. This observation was also confirmed by the fact that the length of the PEO chain of the surfactants studied did not affect the solubilization capacity, for a given tail length, and thus solubilization should not have occurred in the palisade layer or among the PEO heads.
In general, the amount of drug solubilized in a micellar system increases with the increase in temperature. Alkhamis et al. (32) studied the solubilization of the drug gliclazide, a second-generation sulfonylurea used in the treatment of non-insulin dependent diabetes mellitus. The drug solubility was determined as a function of the concentration of different surfactants at 25 and 37°C and, for all the ionic surfactants studied, the solubilization was higher at 37°C than at 25°C. This was attributed to the increase in thermal agitation, which results in an increase in the space available for solubilization in the micelle, in addition to the increase of gliclazide solubility in water at higher temperatures. For the polyoxyethylene nonionic surfactants, the effect of the temperature on the extent of drug solubilization may depend on whether the drug is located inside the hydrophobic core or in the palisade layer. In this same work, the solubility of gliclazide was found to decrease with temperature for the nonionic surfactants studied. Barry and El Eini (30) also observed a significant decrease in the micelle/water molar partition coefficient, PM, obtained for nonpolar steroidal drugs in PEO surfactants solutions when the temperature was increased from 10 to 50°C. The drugs are believed to be located preferentially in the palisade layer, and the increase in temperature causes dehydration of the PEO groups, bringing them closer and consequently reducing the space available for the drugs in this region of the micelle. Nevertheless, the solubility of drugs located preferentially in the inner core of PEO micelles is expected to increase as the temperature is raised, due to micellar growth (23).
The ionic strength can influence significantly the solubilization of a drug in micellar solutions, especially in case of ionic surfactants. The addition of small amounts of salts decreases the repulsion between the similarly charged ionic surfactant head groups, thereby decreasing the cmc and increasing the aggregation number and volume of the micelles. The increase in aggregation number favors the solubilization of hydrophobic drugs in the inner core of the micelle. On the other hand, the decrease in mutual repulsion of the ionic head groups causes closer packing of the ionic surfactant molecules in the palisade layer decreasing the volume available for solubilization of polar drugs. The addition of salts to solutions of PEO nonionic surfactants may also increase the extent of solubilization of hydrophobic drugs because of the increase in aggregation number (17).
The pH of micellar solutions can also show significant influence on the extent of solubilization of drugs, since it may change the equilibrium between ionized and molecular forms of some drugs. LI et al . (33) studied the solubility of the ionized and un-ionized forms of flavopiridol in polysorbate solutions at different pH values. This drug is a weakly basic (pKa = 5.68) derivative of rohitukine that has been developed for breast cancer treatment. The authors observed that the highest total drug solubility was achieved at pH 4.3 where most of the drug was ionized. More recently, Li and Zhao (34) studied the solubilization of flurbiprofen, a non-steroidal antinflamatory drug used in rheumatoid arthritis, in polysorbate solutions at different pH values. This drug is a weak acid, with a pK a of 4.17. It was observed that the drug solubility increases with the increase in pH for pH values over the pK a , due to the increase in the ionized form of the drug. The authors have also proposed an equilibrium-based model to characterize drug-surfactant interactions in pH-controlled systems, reflecting both interactions and interdependence among all drug-containing species: unionized drug in water, ionized drug in water, unionized drug in micelles, and ionized drug in micelles. The model proposed yielded reasonably good estimation when compared to experimental data.
Regarding ionic surfactants, a particular kind of behavior can be observed for the solubility of drugs at different pH values. Enhanced solubility of a drug may be observed at pH values at which the drug is found mostly ionized, when surfactant and drug are oppositely charged. This behavior is a consequence of the electrostatic interactions between the surfactant molecules and the charged drug that causes a decrease in the repulsive forces between the head groups of the surfactant molecules, contributing to the micellization process and thus decreasing the cmc value. In fact, an early study has demonstrated that the drug chlorpromazine can form mixed monomolecular films with phospholipids such as L-α-dipalmitoyl phosphatidylethanolamine and L-α-dipalmitoyl phosphatidyl-choline (35).
More recently, Caetano et al. (36) observed a comicellization phenomenon for the negatively charged surfactant SDS and trifluoperazine, an amphiphilic cationic drug used as antipsychotic and tranquilizer. The authors demonstrated, based on SAXS (Small Angle X-ray Scattering) studies, that the presence of the protonated drug mediates the effect that the counter ion has on the SDS micelle, in such a way that the drug is able to promote micellar surface charge screening. Moreover, the electrostatic interaction between the positively charged drug and the negatively charged SDS must cause a decrease in the repulsive forces between the head groups of the surfactant.
One interesting approach is to combine micellar solubilization with other properties that may be improved in a drug solution. In this context, recently Palma et al. (37) combined the solubilization properties of a surfactant with the ascorbic acid antioxidant property that protects drugs from degradation by light, heat, dissolved oxygen and other radical producing species, by means of synthesizing an ascorbyl-decanoate surfactant. It was observed that micellar solutions of the surfactant obtained significantly improved the solubility of hydrophobic drugs with respect to pure water, by including these molecules in the hydrophobic micellar core, as well as protected them from degradation. It was also observed that the drug solubilization was more effective for the most hydrophobic drugs (Danthron and Griseofulvin) than for more hydrophilic ones (Phenacetin).
A nonionic surfactant that deserves special attention is Cremophor EL (CrEL), which has been used for solubilization of a wide variety of hydrophobic drugs such as anaesthetics, photosensitizers, sedatives, immunosuppressive agents and anticancer drugs. This heterogeneous surfactant is a result of the reaction of castor oil with ethylene oxide, with polyoxyethylene glycerol ricinoleate 35 as the major component identified (38). Formulations containing CrEL have been shown to present important biological side effects, including severe anaphylactic hypersensitivity reactions, hyperlipidaemia, abnormal lipoprotein patterns, aggregation of erythrocytes and peripheral neuropathy (39-44).
One of the most recognized applications of CrEL is the pharmaceutical formulation of paclitaxel, a hydrophobic drug active against several murine tumors that has its development suspended for several years due to solubilization problems. Various studies have shown that CrEL influences the pharmacokinetics of many drugs including paclitaxel that presents a nonlinear disposition when formulated with this surfactant (38). Recently, Sparreboom et al. (45) proposed that the effect of CrEL on paclitaxel pharmacokinetics is associated with micellar solubilization, i.e. encapsulation of the drug within CrEL micelles, with the micelles acting as the principal carrier of paclitaxel in the systemic circulation.
In paclitaxel I.V. infusions, an exceptionally large amount of CrEL is necessary, resulting in important biological events that can lead to serious acute hypersensitivity reactions and neurological toxicity. Therefore, large variety CrEL-free formulation vehicles for paclitaxel are currently in (pre)clinical development, including liposomes, nanocapsules and microspheres (46).
Polymeric Micelles and Drug Delivery
Long-circulating pharmaceuticals and drug carriers represent a growing area of medical and pharmaceutical research. There are several reasons for the search for long-circulating pharmaceuticals and drug carriers, such as:
(i) Long-circulating particles may be used to maintain a required level of a pharmaceutical agent in the blood for extended time intervals for better drug availability. Moreover, long-circulating diagnostic agents are of primary importance for blood pool imaging (47).
(ii) Long-circulating particles of nanoscopic size can slowly accumulate in pathological sites with affected and leaky vasculature (such as tumors, inflammations, and infarcted areas) and improve or enhance drug delivery in those areas. This phenomenon is usually called enhanced permeability and retention effect, EPR, known also as "passive" targeting or accumulation via an impaired filtration mechanism (48,49).
(iii) Prolonged circulation can help to achieve a better targeting effect for specific ligand-modified drugs and drug carriers, since it increases the total quantity of targeted drug/carrier passing through the target, and the number of interactions between the drug and the target (50).
As stated before, micellar systems present some advantages when compared to other drug carriers. For example, micelles can be obtained in an easy and reproducible manner in large scale and specific ligands can be attached to their outer surface in order to optimize the controlled releasing and specificity of pharmacological effect (7). Polymeric carriers might lead to precipitation in water, since the drug-polymer interaction can result in conversion of functional water-soluble groups of the drug into more hydrophobic groups. Micelles, on the other hand, offer a core/shell structure and, therefore, stay water-soluble (51).
According to Kabanov et al. (52), the ideal self-assembling drug delivery system should spontaneously form from drug molecules, carrier components and targeting moieties; their size should be of around 10 nm in order to enable them to penetrate various tissues and even cells; they should be stable in vivo for a sufficiently long period of time without provoke any biological reactions; should release the drug upon contact with target tissues/cells; and the components of the carrier (surfactant molecules) should be easily removed from the body when the therapeutic function is completed.
A very important property of micelles is their size, which is normally around 5 to 100 nm, filling the gap between such drug carriers as individual macromolecules (antibodies, albumin, and dextran) with size below 5 nm, and particles such as liposomes and microcapsules with size of 50 nm and up. The most usual size of a pharmaceutical micelle is between 10 and 80 nm and the optimal cmc value should be in a low millimolar region.
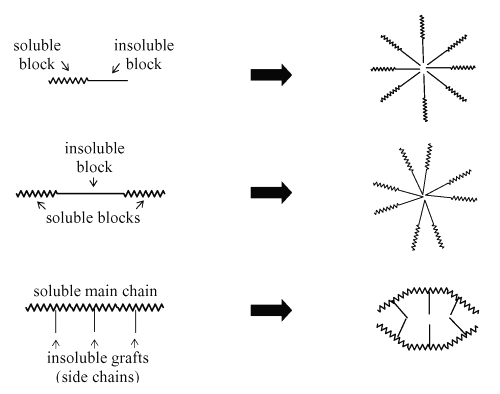
In drug delivery, special attention has been given to the so-called polymeric micelles (5,7,53-57). Polymeric micelles are formed from copolymers consisting of both hydrophilic and hydrophobic monomer units, such as PEO and PPO (polypropylene oxide), respectively. These amphiphilic block co-polymers with the length of the hydrophilic block exceeding the length of the hydrophobic block can form spherical micelles in aqueous solution. The micellar core consists of the hydrophobic blocks and the shell region consists of the hydrophilic blocks (53). The PEO coating has been shown to prevent opsonization and subsequent recognition by the macrophages of the reticuloendothelial system (RES), allowing the micelles to circulate longer and deliver drugs more effectively to the desired sites. (58). Another advantage of polymeric micelles refers to the ease of sterilization via filtration and safety for administration (59, 60). Figure 6 presents a schematic representation of the mechanism of polymeric micelles formation.
Figure 6: Formation of polymeric micelles from different types of amphiphilic block co-polymers (Extracted from Torchillin, 2001).
As aforementioned, micelles are subject to extreme dilution upon intravenous injection into humans. However, the slow dissociation of kinetically stable polymeric micelles allows them to retain their integrity and perhaps drug content in blood circulation above or even below the cmc for some time, creating an opportunity to reach the target site before decaying into monomers (51,61). In addition, some polymeric micelles seems to present better solubilization capacity when compared to surfactant micelles due to the higher number of micelles and/or larger cores of the formers (62).
Besides the solubilization of a drug by physical encapsulation, polymeric micelles can be loaded with hydrophobic molecules that are conjugated or complexed with the polymeric backbone (63). In case of drug conjugates, there should be a cleavage (hydrolysis) of the covalent bond between drug and polymer. Therefore, the release may be dependent on the rate of micellar dissociation, since water diffusion into the hydrophobic micellar core must be restricted, resulting in a sustained drug release (64).
Most studies and applications that have been conducted are based on block copolymers of PEO and PPO blocks, commercially known as Pluronics® (65). Studies for the solubilization of drugs such as haloperidol, indomethacin, doxorubicin (DOX), amphotericin B and digoxin have been reported (52, 66-70) and a parenteral formulation of DOX in these polymeric micelles has entered the phase I of clinical trials in Canada.
More recently, biodegradable block copolymers with polyester core-forming structures have been developed. For example, micelles of PEO-poly(D,L-lactic acid-co-caprolactone) (PEO-PDLLA) have been used to encapsulate paclitaxel and shown similar in vitro toxicity, fivefold increase in maximum tolerable dose and increased efficacy after intraperitoneal injection in murine P388 leukemia model when compared to the standard formulation with Cremophor EL (71).
Polymeric micelles made of poly(ethylene oxide)-b-poly( L -amino acid) (PEO- b -PLAA) has been suggested as synthetic analogs of natural carriers presenting a unique ability for chemical modification, since the free functional groups of PLAA blocks constitute sites to attach drugs. In addition, these PLAA blocks are of increasing interest once they may generate biocompatible monomers after hydrolysis and/or enzymatic degradation (61).
Yokoyama et col. studied PEO- b -poly( L -aspartic acid)-DOX conjugates and, according to the results, the superiority of the block copolymer-drug conjugate over the free drug was a result of the lower toxicity of the former (72-75). Cisplatin (CIS) has also been complexed with PEO-b-poly(Asp), demonstrating increase in cytotoxic concentration against B16 melanoma cells and lower nefrotoxicity (76). In addition, a PEO- b -poly( α -glutamic acid)-CIS complex was investigated and presented greater stability, prolonged circulation in blood stream and improved accumulation in tumor site when compared to the previous complex (77).
Despite the several block copolymer-drug conjugates studies, physical encapsulation of drugs within polymeric micelles offers a great alternative, since conjugation of the drug may lead to changes in the biological properties of the drug and consequently difficult the characterization and regulatory approval of the drug. However, physical encapsulation may present low capacity and/or rapid release of the encapsulated drug (51).
Other alternative that emerges in the field of polymeric micelles refers to polyion complex micelles. Oppositely charged macromolecules, such as peptides and DNA, can complex with the charges of the side chains of some PLAA blocks resulting in the required amphiphilic character for micellization of the complex and leading to stabilization against digestive enzymes such as nucleases (51). These systems seem to be promising and have been receiving significant attention (78-81).
Recently, polymeric micelles incorporating CIS were prepared through polymer-metal complex formation between CIS and poly(ethylene glycol)-poly(glutamic acid) block copolymers, and showed remarkably prolonged blood circulation and effective accumulation in solid tumors (82). Other polyion complex micelles composed of a porphyrin dendrimer and PEG-b-poly(aspartic acid) were evaluated as new photosensitizers for photodynamic therapy in the Lewis lung carcinoma cell line and resulted in reduced dark toxicity of the cationic dendrimer porphyrin, probably due to the biocompatible PEG shell of the micelles (83).
In another work, α-lactosyl-PEG-poly(2(dimethylamino)ethyl methacrylate) block copolymer (lactose-PEG-PAMA) was synthesized to construct a polyion complex micelle-type gene vector potentially useful for selective transfection of hepatic cells, by spontaneously complexion with plasmid DNA encoding luciferase (pGL3-Luc). The lactose-PEG-PAMA-pDNA micelle revealed enhanced transfection compared to the control polyion complex micelle without the ligand (lactose) at a lower pDNA dose (84).
One of the drawbacks of polyion complex micelles is the sensitivity to environment changes such as dilution and ionic strength. To overcome these, polymeric micelles prepared from PEG-poly(a,b,aspartic acid) and the cationic protein trypsin were cross-linked with glutaraldehyde through the Schift base formation, conferring stability to high salt concentrations and increasing the stability of the protein (85).
Micelles, Biological Systems and Micellar Catalysis
The study of cell membranes and of the roles it plays in living cells contributes significantly to the understanding of cellular function. Membranes have been shown to consist of lipids in association with proteins and glycoproteins (16). The present accepted model of a biomembrane is that the phospholipids are organized in a bilayer structure, resulting in a fluid lipid matrix of varying composition and fluidity. Embedded in this matrix are the integral proteins that are able to undergo lateral and rotational diffusion. A wide variety of lipids is found in biological membranes, with the phospholipids being among the most common (86).
Many biological processes occur at membrane surfaces or within their hydrophobic moiety. Owing to the ionic head groups of the lipids, the surface of biological membranes frequently presents a net charge, giving rise to different binding properties of charged and uncharged forms of molecules such as drugs (87-88). In this sense, the relationship between the binding properties of a drug and its active form, as well as its membrane location, deserves attention. Despite the effort aiming at an understanding of drugs mechanism of action at the molecular level, demonstrate by the number of studies on the interaction of drugs with biological membranes, more studies involving model systems are necessary (87,89).
Surfactants have a far-ranging use in membrane studies. Because surfactants are amphiphilic molecules, like lipids, some of the same rules governing lipid behavior also apply to the surfactants. Among the membrane models utilized, micellar systems can be considered an interesting alternative to study the interactions of different compounds with membranes because of the relative simplicity of these systems, and therefore have been used with this purpose (13,20).
Reactions behavior observed at surfactant interfaces are expected to be more representative of many biological reactions than are reactions studied in dilute aqueous solutions (90). In this sense, micellar catalysis of reactions is important because of the parallel with enzymes behavior. Catalysis by both normal micelles and reversed micelles is possible. In normal micelles in aqueous medium, enhanced reaction of the solubilized substrate generally occurs at the micelle-water interface; in reversed micelles in non-polar medium this reaction occurs deep in the inner core (17).
Micellar catalysis in aqueous solution is generally explained in terms of distribution of reactants between water and micelles, with reactions occurring in both media. Therefore, it is possible to treat the rate-surfactant profiles in terms of the concentrations of reactants in the aqueous and micellar pseudo-phases and the rate constants in each pseudo-phase (91).
There are different kinetic models to explain micellar catalytic effects in aqueous medium (92-95). In the pseudo-phase kinetic model (92), the kinetics of a nth order reaction is analyzed by considering the partitioning of the reactants between the two pseudo-phases.
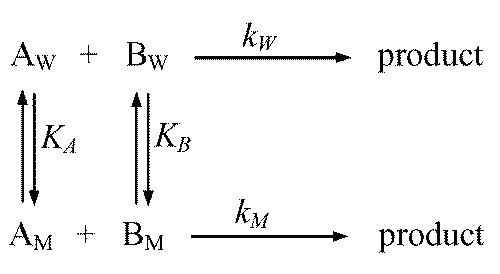
The reactants (A and B) may be distributed as shown in Figure 7.
Figure 7: Schematic representation of a micellar catalyzed reaction according to the pseudo-phase kinetic model. AW, BW and AM, BM correspond to the concentrations of the reactants in the aqueous (W) and micellar (M) phases; KA and KB are the biding constants of the reactants to the micelles, and kW and kM are the rate constants in the aqueous and micellar paths.
Therefore, a quantitative rate expression for a bimolecular reaction can be given by the following equation:
(6)
where kexp is the observed second-order rate constant, PA and PB are the partition coefficients of the reactants A and B , respectively; C is the total surfactant molar concentration minus the cmc; V is the partial molar volume of the surfactant in the micelle and, therefore, CV and (1 - CV ) stand for the volume fractions of the micellar and aqueous phases, respectively. The binding constants ( KA and KB ) are related to the partition coefficient ( P ), as follows:
(7)
For dilute surfactant solutions, where the volume fraction of the micellar phase is small, 1 >> CV and Eq. (6) can be simplified to:
(8)
Utilizing Eq. (7), we can rewrite Eq. (8) as follows:
(9)
In this model, no distinction is made between the various regions of the micelles, although reactions generally occur in the Stern layer, at the micelle/water interface, rather than in the hydrocarbon-like core of the micelle. Nevertheless, the pseudo-phase model explains many features of micellar rate effects and it can be applied, at least qualitatively, to a variety of reactions in colloidal assemblies (96).
Since the binding constant K depends on the extent of hydrophobic bonding between surfactant and substrate, it can be expected that K will increase with increase in the chain length of both the surfactant and the substrate. However, if the hydrophobic group of the substrate is too long, it may be solubilized so deeply in the micelle that access to its reactive site by a reagent in aqueous solution phase is hindered and, therefore, solubilization will inhibit the reaction (17).
The charge of surfactant head group also influences the catalytic power of micelles. Thus, catalysis of some nucleophilic aromatic substitution reactions is more pronounced by dicationic micellar surfactants than by cationic micellar hexadecyltrimethylammonium bromide (96). Yu et al . (97) observed that cationic micelles inhibit, anionic micelles accelerate and nonionic micelles show no appreciable effect on metal ion hydrolysis of p -nitrophenyl picolinate. The higher the electron charge of metal ions, the greater these effects are, indicating that electrostatic interactions are the major contribution for this reaction in micellar solution. In another work, it was observed an increase in the oxidation rate of L(+)arabinose by chromic acid with the addition of SDS and Triton X-100 concentrations. On the other hand, the addition of ammonium, lithium and sodium bromides in SDS micelles resulted in rate decrease (98).
Plots of rate constant versus surfactant concentration often show a maximum at some surfactant concentration above the CMC. One of the reasons for this is that the number of micelles increases with increase in the surfactant concentration. When the number of micelles exceeds that required to solubilize all of the substrate there is a dilution of the substrate concentration per micelle with further increase in surfactant concentration, leading to a reduction in the rate constant. Moreover, the charge surface of an ionic micelle in aqueous solution may cause not only the concentration of an oppositely charged reactant at the micelle-solution interface, but the adsorption of that reactant on it or even the solubilization into micelles, resulting in a decrease in the reactant activity in the solution phase. Therefore, an increase in surfactant concentration over that required to complete solubilization of the substrate may result in a decrease in the rate constant (17).
There is strong evidence to believe that most micelle-catalyzed reactions occur on the surface of the ionic micelles, at or near the charged double layer that surrounds the hydrocarbon core. Typically, reactions between very hydrophobic substrates and hydrophilic anions seem to have lower second-order rate constants in the micellar pseudo phase than in water, because the anions are located in the Stern layer at the micelle/water interface whereas the substrate may be, on average, more deeply in the micelle (96).
Micelles also allow co-solubilization of compounds of very different hydrophobic and hydrophilic character and, as a result, chemical reactions can be developed which otherwise would proceed only with difficulty. An example is the formation of o-phthaldehyde adducts from water-insoluble amines of high molecular mass (99). The presence of micelles can also result in the formation of different reaction products. A diazonium salt in an aqueous micellar solution of sodium dodecyl sulfate, for example, yielded the corresponding phenol from reaction with OH- in the bulk phase, but the corresponding hydrocarbon from material solubilized in the micelles (100).
One interesting approach refers to functional surfactants, which are surfactants containing a reactive residue, usually at the head group, that can be micellized or co-micellized with a chemically inert, non-functional surfactant. In this sense, micelles functionalized with groups that model the amino acid side chains responsible for enzyme activity are generally impressive catalysts (96).
Final Considerations
Considering the importance of micellar systems in the pharmaceutical field and the many applications that it presents, our group have been carrying research on this matter, with special attention to the solubilization of drugs in aqueous micellar solutions. We study the solubilization of model drugs, such as the non-steroidal anti-inflammatory ibuprofen, as well as of potential drugs such as p -substituted benzhydrazides compounds in solutions of different surfactants.
Recently, we investigated the solubilization of ibuprofen (IBU) in micellar solutions of three surfactants possessing the same hydrocarbon tail but different hydrophilic head groups, namely sodium dodecyl sulphate (SDS), dodecyltrimethylammonium bromide (DTAB), and n -dodecyl octa(ethylene oxide) (C 12 E 8 ) (101). The results obtained showed that, irrespective of the surfactant type, the solubility of IBU increases linearly with increasing surfactant concentration, because of the association between the drug and the micelles. Nonionic surfactants were shown to provide a combination of good molar solubilization capacity and high micellar concentration, due to their low cmc , resulting in increased solubility of IBU. On should keep in mind that the low toxicity of nonionic surfactants makes them particularly interesting for solubilization and drug delivery purposes. In addition to these studies, Small Angle X-ray Scattering (SAXS) studies on the interaction of ibuprofen with micelles of SDS, DTAB and C 12 E 8 are in progress, aiming at a deeper understanding on the nature of these interactions as well as on the properties of the aggregates obtained.
The p-substituted benzhydrazides are hydrophobic compounds synthesized by Taveres et col. that represent potential drugs with anti-staphylococci and anti-trypanosome activities. Quantitative Structure-Activity Relationships (QSAR) studies and experimental results have shown a dependency between biological activity and hydrophobicity of these compounds, with the more hydrophobic molecules presenting higher activity (102,103). However, the more hydrophobic the compound, the more difficult the solubilization in the culture media used for activity determination. Therefore, the possibility of micellar solubilization of these molecules should contribute to more precise determinations of biological activity. We are currently carrying on experiments on the solubilization of p -substituted benzhydrazides in aqueous micellar solutions of n -dodecyl octaethylene oxide (C 12 E 8 ) and n -hexadecyl octaethylene oxide (C 16 E 8 ), two nonionic surfactants possessing the same hydrophilic head groups but different hydrophobic tails.
Acknowledgements
Carlota Rangel-Yagui is grateful for the Post-Doctoral fellowship and financial support from FAPESP (Fundação de Amparo à Pesquisa no Estado de São Paulo). We acknowledge CAPES (Coordenação de Aperfeiçoamento de Pessoal de Nível Superior -Brazil) and CNPq (Conselho Nacional de Desenvolvimento Científico e Tecnolόgico - Brazil) for financial support.
References
Mall, S., Buckton, G., Rawlins, D.A. Dissolution behaviour of sulphonamides into sodium dodecyl sulphate micelles: A thermodynamic approach. J Pharm Sci, 85(1):75-78, 1996.
Allen, T.M., Hansen, C.B., Menenez, D.E.L. Pharmacokinetics of long-circulating liposomes. Adv. Drug Deliv Rev, 16:267–284, 1995.
Canto, G.S., Dalmora, S.L., Oliveira, A.G. Piroxicam encapsulated in liposomes: characterization and in vivo evaluation of topical anti-inflammatory effect. Drug Dev Ind Pharm, 25:1235-1239, 1999.
Gref, R., Minamitake, Y., Peracchia, M.T., Trubetskoy, V.S., Torchilin, V.P., Langer, R. Biodegradable long-circulating polymeric nanospheres. Science, 263:1600–1603, 1994.
Jones, M.C., Leroux, J.C. Polymeric micelles — a new generation of colloidal drug carriers. Eur J Phar Bio Pharm, 48:101–111, 1999.
Torchilin, V.P., Trubetskoy, V.S., Whiteman, K.R., Caliceti, P., Ferruti, P., Veronese, F.M. New synthetic amphiphilic polymers for steric protection of liposomes in vivo. J Pharm Sci, 84:1049-1053, 1995.
Torchilin, V.P. Structure and design of polymeric surfactant-base drug delivery systems. J Control Rel, 73:137-172, 2001.
Martin, A. Physical Pharmacy, 4ed., Williams and Wilkins, Baltimore, USA, pp 396–398, 1993.
Hunter, R.J. Introduction to Modern Colloid Science. Oxford University Press, Oxford, 1993.
Ford, J.M., Hait, W.N. Pharmacology of drugs that alter multidrug resistance in cancer. Pharmacol Rev, 42(3):155-199, 1990.
Chakraborty, H., Banerjee, R., Sarkar, M. Incorporation of NSAIDS in micelles: implication of structural switchover in drug-membrane interaction. Biophys Chem, 104(1):315-325, 2003.
Ferreira, H., Lucio, M., De Castro, B., Gameiro, P., Lima, J.L.F.C., Reis, S. Partition and location of nimesulide in EPC liposomes: a spectrophotometric and fluorescence study. Anal Bioanal Chem, 377(2):293-298, 2003.
Fresta, M., Guccione, S., Beccari, A.R., Furneri, P.M., Puglisi, G. Combining molecular modeling with experimental methodologies: Mechanism of membrane permeation and accumulation of ofloxacin. Bioorg Med Chem, 10(12):3871-3889, 2002.
Israelachvili, J.N., Mitchell, D.J., Ninham, B.W. Theory of self-assembly of hydrocarbon amphiphiles into micelles and bilayers. J Chem Soc Far Trans II, 72:1525-1568, 1976.
Suwalsky, M., Hernandez, P., Villena, F., Aguilar, F., Sotomayor, C.P. Interaction of the anticancer drug tamoxifen with the human erythrocyte membrane and molecular models. Zeitschrift fur Naturforschung C - J Biosciences, 53(3-4):182-190, 1998.
Jones, M.N., Chapman, D. Micelles, monolayers and biomembranes. Wiley-Liss, New York, 1995.
Rosen, M.J. Surfactants and interfacial phenomena, 2ed., John Wiley & Sons, New York, 1989.
Chevalier, Y., Zemb, T. The structure of micelles and microemulsions. Rep Prog Phys, 53:279-371, 1990.
Tanford, C. The hydrophobic effect: Formation of micelles and biological membranes. Wiley, New York, 1980.
Israelachvili, J.N. Intermolecular and surface forces, 2ed., Academic Press, London, 1991.
Hiemenz, P.C., Rajagopalan, R. Principles of colloid and surface chemistry, 3ed. Marcel Decker, New York, 1997.
Puvvada, S., Blankschtein, D. Molecular-thermodynamic approach to predict micellization, phase behavior, and phase separation of micellar solutions. I. Application to nonionic surfactants. J Chem Phys, 92(6):3710-3724. 1990.
Florence, A.T., Attwood, D. Physicochemical Principles of Pharmacy, 3rd ed. The MacMillan Press, London, 2003.
Attwood, D., Florence, A.T. Surfactant systems: Their chemistry, pharmacy and biology. Chapman and Hall, New York, 1983.
Alvarez-Núñez, F.A., Yalkowsky, S.H. Relationship between polysorbate 80 solubilization descriptors and octanol-water partition coefficient of drugs. Int J Pharm, 200:217-222, 2000.
Yokoyama, M. Block copolymers as drug carriers. CRC Crit Rev Ther Drug Carrier Syst, 9:213-248, 1992.
Dutt, G.B. Rotational diffusion of hydrophobic probes in Brij-35 micelles: Effect of temperature on micellar internal environment. J Phys Chem B, 107:10546-10551, 2003.
Mukerjee, P., Cardinal, J.R. Benzene derivatives and naphthalene solubilized in micelles. Polarity of microenvironment, location and distribution in micelles, and correlation with surface activity in hydrocarbon-water systems. J Phys Chem, 82(14):1620-1627, 1978.
Krishna, A.K., Flanagan, D.R. Micellar solubilization of a new antimalarial drug, b-arteether. J Pharm Sci, 78(7):574-576,1989.
Barry, B.W., El Eini, D.I.D. Solubilization of hydrocortisone, dexamethasone, testosterone and progesterone by long-chain polyoxyethylene surfactants. J Pharm Pharmac, 28:210-218, 1976.
Ong, J.T.H., Manoukian, E. Micellar solubilization of timbesone acetate in aqueous and aqueous propylene glycol solutions of nonionic surfactants. Pharm Res, 3(11):704-708, 1988.
Alkhamis, K.A., Allaboun, H., Al-Momani, W.Y. Study of the solubilization of gliclazide by aqueous micellar solutions. J Pharm Sci, 92(4):839-846, 2003.
Li, P., Tabib, E., Yalkowsky, S.H. Solubilization of ionized and un-ionized flavopiridol by ethanol and polysorbate 20. J Pharm Sci, 88(5):507-509, 1999.
Li, P., Zhao, L. Solubilization of flurbiprofen in pH-surfactant solutions. J Pharm Sci, 92(5):951-956, 2003.
Zografi, G., Auslander, D.E. Surface activity of chlorpromazine and chlorpromazine sulfoxide in the presence of insoluble monomolecular films. J Pharm Sci, 54(9):1313-1318, 1965.
Caetano, W., Gelamo, E.L., Tabak, M., Itri R. Chlorpromazine and sodium dodecyl sulphate mixed micelles investigated by Small Angle X-Ray Scattering. J Colloid Interf Sci, 248:149-157, 2002.
Palma, S., Manzo, R.H., Allemandi, D., Fratoni, L., Lo Nostro, P. Drug solubilization in ascorbyl-decanoate micellar solutions. Colloid Surf A, 212:163-173, 2003.
Gelderblom, H., Verweij, J., Nooter, K., Sparreboom, A. Cremophor EL: the drawbacks and advantages of vehicle selection for drug formulation. Eur J Cancer, 37:1590-1598, 2001.
Adams, J.D., Flora, K.P., Goldspiel, B.R., Wilson, J.W., Arbuck, S.G.I. Taxol: a history of pharmaceutical development and current pharmaceutical concerns. J Natl Cancer Inst Monogr, 15:141-147, 1993.
Watkins, J., Ward, A.M., Appleyard, T.N. Adverse reactions in intravenous anaesthetic induction agents. Br Med J, 2:1084-1085, 1997.
Huttel, M.S., Olesen, A.S., Stoffersen, E. Complement-mediated reactions to diazepam with cremophor as solvent (steroid MR). Br J Anaesth 52(1):77-79, 1980.
Kongshaug, M., Cheng, L.S., Moan, J., Rimington, C. Interaction of Cremophor-EL with human plasma. Int J Biochem, 23(4):473-478, 1991.
Woodburn, K., Kessel, D. The alteration of plasma-lipoproteins by Cremophor EL. J Photoch Photobiol B, 22(3):197-201, 1994.
Windebank, A.J., Blexrud, M.D., Degroen, P.C. Potential neurotoxicity of the solvent vehicle for cyclosporine. J Pharmacol Exp Ther, 268(2):1051-1056, 1994.
Sparreboom, A., van Zuylen, L., Brouwer, E., Loos, W.J., de Bruijn, P., Gelderblom, B., Pillay, M., Nooter, K., Stoter, G., Verweij, J. Cremophor EL-mediated alteration of paclitaxel distribution in human blood: Clinical pharmacokinetic implications. Cancer Res, 59(7):1454-1457, 1999.
van Zuylen, L., Verweij, J., Sparreboom, A. Role of formulation vehicles in taxane pharmacology. Inv New Drug, 19(2):125-141, 2001.
Torchilin, V.P., Trubetskoy, V.S. Which polymers can make nanoparticulate drug carriers long-circulating? Adv Drug Deliv Rev, 16:141–155, 1995.
Palmer, T.N., Caride, V.J., Caldecourt, M.A., Twickler, J., Abdullah, V. The mechanism of liposome accumulation in infarction. Biochim Biophys Acta,797:363–368, 1984.
Maeda, H., Wu, J., Sawa, T., Matsumura, Y., Hori, K. Tumor vascular permeability and the EPR effect in macromolecular therapeutics: a review. J Contr Rel, 65:271–284, 2000.
Torchilin, V.P. How do polymers prolong circulation time of liposomes? J Liposome Res, 6:99–116, 1996.
Lavasanifar, A., Samuel, J., Kwon, G.S. Poly(ethylene oxide)-block-poly(L-amino acid) micelles for drug delivery. Adv Drug Deliv Rev, 54:169-190, 2002.
Kabanov, A.V., Batrakova, E.V., Melik-Nubarov, N.S., Fedoseev, N.A., Dorodnich, T.Y., Alakhov, V.Y., Chekhonin, V.P., Nazarova, I.R., Kabanov, V.A. A new class of drug carriers; micelle poly(oxyethylene)-poly(oxypropylene) block copolymers as microcontainers for drug targeting from blood to brain. J Contr Rel, 22:141–158, 1992.
Kwon, G.S., Kataoka, K. Block copolymer micelles as long-circulating carriers for delivery of therapeutic and diagnostic agents. Adv Drug Deliv Rev, 16:295-309, 1995.
Inoue, T., Chen, G.H., Nakamae, K., Hoffman, A.S. An AB block copolymer of oligo(methyl methacrylate) and poly(acrylic acid) for micellar delivery of hydrophobic drugs. J Control Rel 51(2-3):221-229, 1998.
Discher, B.M., Won, Y.Y., Ege, D.S., Lee, J.C.M., Bates, F.S., Discher, D.E., Hammer, D.A. Polymersomes: Tough vesicles made from diblock copolymers. Science, 284(5417):1143-1146, 1999.
Rapoport, N., Marin, A., Luo, Y., Prestwich, G.D., Muniruzzaman, M. Intracellular uptake and trafficking of pluronic micelles in drug-sensitive and MDR cells: Effect on the intracellular drug localization. J Pharm Sci, 1:157-170, 2002.
Soo, P.L., Luo, L., Maysinger, D., Eisenberg, A. Incorporation and release of hydrophobic probes in biocompatible polycaprolactone-block-poly(ethylene oxide) micelles: implications for drug delivery. Langmuir, 18:9996-10004, 2002.
Lee, J.H., Lee, H.B., Andrade, J.D. Blood compatibility of polyethylene oxide surfaces. Prog Polym Sci, 20(6):1043-1079, 1995.
Kwon, G.S., Kataoka, K. Block copolymer micelles as long circulating drug vehicles. Adv Drug Deliv Rev, 16:295–309, 1995.
Kwon, G.S., Okano, T. Polymeric micelles as new drug carriers. Adv Drug Deliv Rev, 21:107–116, 1996.
Adams, M.L., Lavasanifar, A., Kwon, G.S. Amphiphilic block copolymers for drug delivery. J Pharm Sci, 92(7):1343-1355, 2003.
Gadelle, F., Koros, W.J., Schechter, R.S. Solubilization of aromatic solutes in block copolymers. Macromolecules, 28:4883–4892, 1995.
Kataoka, K., Harada, A., Nagasaki, Y. Block copolymer micelles for drug delivery: design,characterization and biological significance. Adv Drug Deliv Rev, 47:113–131,2001.
Li, Y., Kwon, G.S. Methotrexate esters of poly(ethylene oxide)-block-poly(2-hydroxyethyl--aspartamide). Part I: effects of the level of methotrexate conjugation on the stability of micelles and on drug release. Pharm Res, 17:607–611, 2000.
Mortensen, K. PEO-related block copolymer surfactants. Coll Surf A, 183-185:277-292, 2001.
Kabanov, A.V., Chekhonin, V.P, Alakhov, V.,. Betrakova, E.V, Lebedev, A.S., Mellik-Nubarov, N.S., Arzakov, S.A., Levashov, A.V., Morozov, G.V., Severin, E.S., Kabanov, V.A. The neuroleptic activity of haloperidol increases after its solubilization in surfactant micelles. FEBS Lett, 258:343–345, 1989.
Lin, S., Kawashima, Y. Kinetic studies on the stability of indomethacin in alkaline aqueous solutions containing poly(oxyethylene)poly(oxypropylene) surface active block copolymers. Pharm Acta Helv, 60:345–350, 1985.
Baratkova, E.V., Dorodnich, T.Y., Klinskii, E.Y., Kliushnenkova, E.N., Shemchukova, O.B., Goncharova, O.N., Arjakov, V.Y., Alakhov, V., Kabanov, A.V. Anthracycline antibiotics noncovalently incorporated into the block copolymer micelles: in vivo evaluation of anticancer activity. Br J Cancer, 74:1545–1552, 1996.
Forster, D., Washington, C., Davis, S.S. Toxicity of solubilized and colloidal amphotericin B formulations to human erythrocytes. J Pharm Pharmacol, 40:325–328, 1988.
Baratkova, E.V., Miller, D.W., Li, S., Alakhov, V., Kabanov, A.V., Elmquist, W.F. Pluronic P85 enhances the delivery of digoxin to the brain: in vitro and in vivo studies. J Pharmacol Exp Ther, 269:551– 557, 2001.
Zhang, X., Burt, H.M., Von Hoff, D., Dexter, D., Mangold, D., Degen, D., Oktaba, M., Hunter, W.L. An investigation of the antitumour activity and biodistribution of polymeric micellar paclitaxel. Cancer Chemother Pharmacol, 40:81–86, 1997.
Yokoyama, M., Kwon, G.S., Okano, T., Sakurai, Y., Sato, T., Kataoka, K. Preparation of micelle-forming polymer–drug conjugates. Bioconjug Chem, 3:295– 301, 1992.
Kwon, G.S., Yokoyama, M., Okano, T., Sakurai, Y., Kataoka, K. Biodistribution of micelle-forming polymer–drug conjugates. Pharm Res, 10:970–974, 1993.
Kataoka, K., Kwon, G.S., Yokoyama, M., Okano, T., Sakurai, Y. Polymeric micelles as novel drug carriers and virus mimicking vehicles. In: J. Kahovec (Ed), Macromolecules, 1992(VSP):267–276, 1993.
Yokoyama, M., Okano, T., Sakurai, Y., Ekimoto, H., Shibazaki, C., Kataoka, K. Toxicity and antitumor activity against solid tumors of micelle-forming polymeric anticancer drug and its extremely long circulation in blood. Cancer Res, 51:3229–3236, 1991.
Mizumura, Y., Matsumura, Y., Hamaguchi, T., Nishiyama, N., Kataoka, K., Kawaguchi, T., Saito, T., Kakizoe, T. Cisplatin-incorporated polymeric micelles reducing nephrotoxicity, while maintaining antitumor activity. Jpn J Cancer Res, 92:328–336, 2001.
Nishiyama, N., Kato, Y., Sugiyama, Y., Kataoka, K. Development of cisplatin-loaded polymeric micelles with a prolonged circulation in the bloodstream and an enhanced accumulation in the solid tumor. Proceedings of the 20th International Symposium on Controlled Release of Bioactive Compounds, 28:5155–5156, 2001.
Kabanov, A.V., Kabanov, V.A. Interpolyelectrolyte and block ionomer complexes for gene delivery: physico-chemical aspects. Adv Drug Deliv Rev, 30:49–60, 1998.
Lemieux, P., Vinogradov, S.V., Gebhart, C.L., Guerin, N., Paradis, G., Nguyen, H.K., Ochietti, B., Suzdaltseva, Y.G., Baratkova, E.V., Bronich, T.K., St-Pierre, Y., Alakhov, V.Y., Kabanov, A.V. Block and graft copolymers and nanogel copolymer networks for DNA delivery into cell. J Drug Target, 8: 91–105, 2000.
Itaka, K., Yamauchi, K., Harada, A., Nakamura, K., Kawaguchi, H., Kataoka, K. Polyion complex micelles from plasmid DNA and poly(ethylene glycol)-poly(L-lysine) block copolymer as serum tolerable polyplex system: physicochemical properties of micelles relevant to gene transfection efficiency. Biomaterials, 24(24):4495-4506, 2003.
Berret, J.F., Vigolo, B., Eng, R., Herve, P., Grillo, I., Yang, L. Electrostatic self-assembly of oppositely charged copolymers and surfactants: A light, neutron, and X-ray scattering study, Macromolecules, 37(13):4922-4930, 2004.
Nishiyama, N., Okazaki, S., Cabral, H., Myiamoto, M., Kato, Y., Sugiyama, Y., Nishio, K., Matsumura, Y., Kataoka, K. Novel cisplatin-incorporated polymeric micelles can eradicate solid tumours in mice. Cancer Res, 63(24):8977-8983, 2003.
Zhang, G.D., Harada, A., Nishiyama, N., Jiang, D.L., Koyama, H., Aida, T., Kataoka, K. Polyion complex micelles entrapping cationic dendrimer porphyrin: effective photosensitizer for photodynamic therapy of cancer. J Control Rel, 93(2):141-150, 2003.
Wakebayashi, D., Nishiyama, N., Yamasaki, K., Itaka, K., Kanayama, N., Harada, A., Nagasaki, Y., Kataoka, K. Lactose-conjugated polyion complex micelles incorporating plasmid DNA as a targetable gene vector system: their preparation and gene transfecting efficiency against cultured HepG2 cells. J Control Rel, 95(3):653-664, 2004.
Jaturanpinyo, M., Harada, A., Yuan, X.F., Kataoka, K. Preparation of bionanoreactor based on core-shell structured polyion complex micelles entrapping trypsin in the core cross-linked with glutaraldehyde. Bioconjugate Chem, 15(2):344-348, 2004.
Yeagle, P.L. The membranes of cells, 2nd ed. New York: Academic Press, 1993.
Caetano, W., Tabak, M. Interaction of chlorpromazine and trifluoperazine with ionic micelles: electronic absorption spectroscopy studies. Spectrochim. Acta A. 55(12):2513-2528, 1999.
Louro, S.R.W., Nascimento, O.R., Tabak, M. Charge-dependent and ph-dependent binding-sites for dibucaine in ionic micelles - a fluorescence study. Biochim Biophys Acta, 1190:319-328, 1994.
Yushmanov, V.E., Perussi, J.R., Imasato, A.C., Rugiero, M. Tabak, M. Ionization and binding equilibria of papaverine in ionic micelles studied by H-1-NMR and optical-absorption spectroscopy Biophys Chem, 52:157-163, 1994.
Price, S.E., Jappar, D., Lorenzo, P., Saavedra, J.E., Hrabie, J.A., Davies, K.M. Micellar catalysis of nitric oxide dissociation from diazeniumdiolates. Langmuir, 19:2096-2102, 2003.
Arikan, B., Tunçay, M. Micellar effects and reactant incorporation in reduction of toluidine blue by ascorbic acid. Dyes and Pigments. 64:1-8, 2005.
Martinek, K., Yatsimirskii, A.K. Levashov, A.V., Berezin, I.V. In: Micellization, Solubilization and Microemulsions, Ed: Mittal, K.L., Plenum, New York, vol.2, p.489, 1977.
Menger, F.M., Portnoy, C.E. On chemistry of reactions proceeding inside molecular aggregates. J Am Chem Soc, 89(18): 4698-&, 1967.
Piszkiewicz, D. Cooperativity in bimolecular micelle-catalyzed reactions - inhibition of catalysis by high-concentrations of detergent. J Am Chem Soc, 99(23):7695-7697, 1977.
Dash, A.C., Prusti, J., Pradhan, J., Das, P.K. Micellar effects upon the reactions of complex-ions in solution .4. Kinetics of aquation and base hydrolysis of some cis-(chloro)(amine)bis (ethylenediamine)cobalt(III) complexes in the presence of neutral and anionic surfactants in an aqueous-medium. J Chem Soc Far Trans, 86(3):507-510, 1990.
Carreto, M.L., Rubio, S., Pérez-Bendito, D. Organic microheterogeneous systems in kinetic analysis. Self assembled systems – A review. Analyst, 121:33R-44R, 1996.
Yu, X.Q., Jiang, B.Y., Cheng, S.Q., Huang, Z., Zeng, X.C. Comparative reactivities of metal cation-catalyzed hydrolysis of p-nitrophenyl picolinate in micellar solutions. J Disp Sci Technol, 24(6):761-765, 2003.
Kabir-ud-Din, Morshed, A., Khan, Z. Oxidative degradation of L(+)arabinose by chromium (VI) in absence and presence of sodium dodecyl sulphate and TX-100 micelles. Oxid Commun, 26(1):59-71, 2003.
Memon, M.H., Worsfold, P.J. The use of microemulsions in flow-injection analysis - spectrofluorimetric determination of primary amines. Anal Chim Acta, 183:179-185, 1986.
Abe, M. Suzuki, N., Ogino, K. Replacement reaction of diazonium salt in the presence of sodium dodecyl-sulfate micelles. J Coll Interface Sci, 93:285-288, 1983.
Rangel-Yagui, C. O., Hsu, H.W.L., Pessoa-Jr, A., Tavares, L.C., Micellar solubilization of ibuprofen – The influence of surfactant head on the extent of solubilization. RBCF – Braz J Pharm Sci, 2004. (In Press)
Furlanetto, M., Masunari, A., Tavares, L. C. Validation of carbonyl group absorption frequency , Vc=o, as a structural descriptor for QSAR/QSPR studies In: 15th European Symposium on Quantitative Structure-Activity Relationships & Molecular Modeling, 2004, Istanbul. Program & Abstracts , p.142, 2004.
Masunari, A., Rezende, P., Tavares, L. C. QSAR studies of nifuroxazide analogs with antimicrobial activity against multidrug-resistant Staphylococcus aureus In: 15th European Symposium on Quantitative Structure-Activity Relationships & Molecular Modeling, 2004, Istanbul. Program & Abstract, p.123, 2004.
Corresponding Author: Dr. Carlota de Oliveira Rangel Yagui, Av. Prof. Lineu Prestes, 580 – Bloco 16, CEP: 05508-950 – São Paulo, SP. corangel@usp.br
Published by the Canadian Society for Pharmaceutical Sciences.
Copyright © 1998 by the Canadian Society for Pharmaceutical Sciences.
http://www.cspscanada.org