J Pharm Pharmaceut Sci
(www.cspscanada.org) 8(2):335-339, 2005
Antifungal activity of drimane sesquiterpenes
from Drimys brasiliensis using
bioassay-guided fractionation
Angela
Malheiros1, Valdir Cechinel Filho1, Clarisse B.
Schmitt2, Rosendo A. Yunes2,
Andrea Escalante3, Laura Svetaz3, Susana Zacchino3,
Franco Delle Monache4
1Núcleo de Investigações
Químico-Farmacêuticas, Universidade do Vale do Itajaí, Itajaí, SC, Brazil; 2Curso
de Pós-Graduação em Química, Universidade Federal de Santa Catarina,
Florianópolis, SC, Brazil; 3Farmacognosia,
Facultad de Ciencias Bioquímicas y Farmacéuticas, Universidad Nacional de
Rosario, Suipacha Rosario, Argentina; 4Centro Chimica Recettori,
Rome, Italy
Received November 19, 2004, Revised December 21,
2004, Accepted June 30, 2005, Published August 15, 2005.
Corresponding author:
Dr. Angela
Malheiros, Núcleo de Investigações Químico-Farmacêuticas/CCS, Universidade do
Vale do Itajaí, 88302-202, Itajaí, SC, Brazil, Fax: 55-47-341 7601, Email:
angela@univali.br
PDF
version
ABSTRACT. Purpose. This study describes the antifungal effect
of extracts and compounds isolated from Drimys
brasiliensis acting against dermatophytes. Methods. The activities were evaluated by using the microbroth
dilution method. Results.
Bioassay-guided fractionation of the most active extract from the bark (CHCl3)
led to the isolation of the sesquiterpene drimanes polygodial,
1-b-(p-methoxycinnamoyl)-polygodial, drimanial and 1-b-(p-cumaroyloxy)-polygodial, which were selectively active against Epidermophyton floccosum and Tricophyton rubrum. Conclusions. The selective antifungal activity reported in this
paper for drimanes isolated from D. brasiliensis opens the possibility that they could be helpful for the developing of
new antifungal agents for treating the difficult to eradicate dermatomycoses
produced by E. floccosum.
INTRODUCTION
Due to the increasing number of immuno-compromised
individuals, fungal infections have increased in the last two decades,
affecting millions of people worldwide
(1). Among them, opportunistic systemic mycoses are associated with high rates
of death (2) and skin fungal infections, although not life threatening, are
very difficult to eradicate (3). They produce a variety of problems, such as
Athletes’s foot and nail infections, leading to the debilitation of the
patients’ quality of life, with the additional danger that they can spread to
other areas of the body and to other individuals (4).
Although there appears to be
many drugs for the treatment of superficial mycoses, there are in fact a
limited number of efficacious antifungal drugs (5). They possess a series of
limitations such as undesirable side effects or rapid development of resistance
and, as a consequence, new antifungal agents are still needed to improve the treatment of
superficial fungal infections (6,7).
In the course of our screening
program for the detection of antifungal compounds, we found in preliminary
assays that CHCl3 extract of Drymis brasiliensis possesses
antifungal activity. These effects were related to the presence of polygodial (1), the major compound present in plant
of this genus (8,9), which has been reported as antifungal in previous studies
(10,11).
In order to detect
some new antifungal compounds with drimane skeleton in extracts of Drymis
brasiliensis, we report here the bioassay-guided fractionation of
chloroform and methanol extracts of different parts of the plant by using the
microbroth dilution method.
MATERIAL
AND METHODS
Plant
material
Barks, stems and leaves of Drimys brasiliensis were collected in
Rancho Queimado, state of Santa
Catarina, Brazil,
in December 1998. The plant was identified as Drimys brasiliensis Miers subsp. sylvatica (Saint Hilaire)
Ehrendofer L Gott sb by Dr. Ademir Reis (Departament of Botany, Universidade
Federal de Santa Catarina) and a voucher specimen was deposited in the Barbosa
Rodrigues Herbarium (Itajaí - SC), under number VC Filho 010.
Preparation
of extracts
Barks of Drimys brasiliensis (1.85 Kg) were dried at 40oC for two
days, powdered and succesively extracted with chloroform and MeOH at room
temperature for 10 days each. The extracts were concentrated under reduced
pressure, giving residues of 30.2 g and 60.6 g, respectively. Stems (700 g)
were processed in the same way as barks, giving 17.6 g and 14.8 g of CHCl3
and MeOH extracts respectively. From 250 g of leaves were obtained similarly
24.5 g and 10.6 g of CHCl3 and MeOH extracts respectively.
Isolation and identification
of drimanes from CHCl3 extract (barks)
Part of the bark extract
(23 g) was subjected to column chromatography (qi
4,5 cm), packed with silica gel 60-230 mesh (220 g), and eluted with hexane
gradually enriched in ethyl acetate and ethanol. 75 fractions of 100 ml each
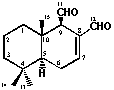
were collected. Polygodial (1) was
obtained from fractions 28-32, [elution solvent Hex-AcOEt (9:1)] and was
further purified by repeated chromatographies, giving 3.1 g yield (0.22 % in
relation to dry plant).
Fraction
48-49, eluted with hexane:ethyl acetate 6:4 (2.3 g), was submitted to column
chromatography (qi 3, 0 cm), packed with silica gel (46 g), eluted with hexane and
gradually enriched with ethyl acetate and ethanol. 51 sub-fractions of 25 mL
each were collected. Sub-fractions 14-18, eluted with hexane:ethyl acetate
7.5:2.5 (198.4 mg) were rechromatographed as indicated above, eluted with
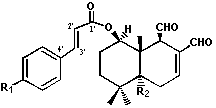
hexane:ethyl acetate 6:4, yielding 12 mg of 1-b-(p-methoxycinnamoyl)-polygodial
(2) (8.5 X 10-4 % in relation to dry plant).
Fraction
50-54, eluted with hexane:ethyl acetate 3:7 (5.5 g), was submitted to column
chromatography (qi 2,5 cm), packed with silica gel (48 g), eluted with hexane and
gradually enriched with ethyl acetate and ethanol. 72 sub-fractions of 25 mL
each were collected. From the subfraction 16-25, eluted with hexane:ethyl
acetate 6:4, 1-b-(p-cumaroyloxy)-polygodial was obtained (187 mg yield, 0.013 % in
relation to dry plant). Finally, from sub-fraction 30-42, eluted with
hexane:ethyl acetate 6:4, drimanial (3) was obtained (3.3 g yield, 0.23
% in relation to dry plant). All the compounds were identified on basis of
their spectral data in comparison with to those of literature [8,9] and direct
comparison with authentic samples.
Microorganisms and media
For the
antifungal evaluation, strains from the American Type Culture Collection
(ATCC), Rockville, MD, USA and CEREMIC (C), Centro de Referencia Micológica,
Facultad de Ciencias Bioquímicas y Farmacéuticas, Suipacha 531-(2000)-Rosario,
Argentina were used: Microsporum canis C 112,
Epidermophyton floccosum C 114, Trichophyton rubrum C 110, T. mentagrophytes ATCC 9972 and Microsporum gypseum C 115. Strains
were grown on Sabouraud-chloramphenicol agar slants for 48 h at 30 oC,
maintained on slopes of Sabouraud-dextrose agar (SDA, Oxoid) and subcultured
every 15 days to prevent pleomorphic transformations. Inocula of cell or spore
suspensions were obtained according to reported procedures and adjusted to 105 cells/spores with
colony forming units (CFU) /ml (12).
Antifungal susceptibility testing
Minimal Inhibitory
Concentration (MIC) of each extract or compound was determined by using broth
microdilution techniques according to the guidelines of the National Committee
for Clinical Laboratory Standards for yeasts (M27-A2) (13) and for filamentous
fungi (M 38 A) (14). MIC values were determined in RPMI 1640 (Sigma, St Louis, Mo,
USA) buffered
to pH 7.0 with MOPS. The
starting inocula were 1x105 to 5x105 CFU/ml. Microtiter trays
were incubated at 35 oC for yeasts and hialohyphomycetes and at
28-30 oC for dermatophyte strains in a moist, dark chamber, and MICs
were visually recorded at 48 h for yeasts, and at a time according to the
control fungus growth, for the rest of fungi.
For the assay, stock solutions of extracts were two-fold diluted with
RPMI 1000-1 µg/ml (final volume = 100 µl) and a final DMSO concentration
≤ 1%. A volume of 100 µl of inoculum suspension was added to each well
with the exception of the sterility control where sterile water was added to
the well instead. Pure compounds were tested from 100 - 1 µg/ml. MIC was
defined as the minimum inhibitory concentration of extract or pure compound
which resulted in total inhibition of the fungal growth. ketoconazole,
terbinafine and amphotericin B were used as positive controls.
RESULTS
AND DISCUSSION
Results of the antifungal
activities of chloroform and methanol extracts of bark, stems and leaves of Drymis
brasiliensis are showed in Table 1.
CHCl3
extracts of the three parts of the plant showed MIC values between 12.5 -1000 µg/ml
against all dermatophytes tested. Chloroform extract of the bark was the most
promising one, with MICs ranging from 12.5 to 100 µg/ml, and being E.
floccosum the most sensitive species (MIC 12.5 µg/ml).
The bioassay-guided
fractionation of CHCl3 extract of barks led to the isolation of the
antifungal drimane polygodial (1), 1-b-(methoxycinnamoyl)-polygodial
(2) and 1-b-(p-cumaroiloxy)-polygodial (4) together with the inactive drimanial
(3) (table 2).
Compounds
1 and 2 have been previously isolated
as antinociceptive from a highly related species Drymis winteri (8,9). In addition polygodial was previously
isolated from Polygonum hydropiper (13), Warburgia ugandensis and Warburgia
stuhlmanii, exhibiting insect
antifeedant and antimicrobial activities (14,15) Pseudowintera colorata
(16) and Polygonum punctatum (17) exhibit antimicrobial activities.
Regarding the reported antifungal activity, polygodial previously showed potent
antifungal activities against yeasts and filamentous fungi being its activity
due to the structural disruption of cell membranes (18, 19)
In
this work we found that polygodial inhibit the growth of Trichophyton rubrum C 110 (see Microorganisms and Media above) with
MIC 25 g/ml and Epidermophyton
floccosum C 114 with MIC 3 µg/ml. It was not active against the
dermatophytes Trichophyton mentagrophyes
ATCC 9972, Microsporum canis C112 and
Microsporum gypseum C 115 up to 100 µg/ml.
Previously
reported antifungal activity of polygodial (20) showed lower MICs (0.78 and
3.13 mg/ml respectively) against the dermatophytes T. rubrum ATCC 28188 and T.
mentagrophytes ATCC 28185 and 18748. No results were reported against the
rest of dermatophytes Epidermophyton
floccosum, Microsporum canis or M. gypseum. A comparison of the previous work and ours with respect
to the activities of polygodial against T.
rubrum and T. mentagrophytes may
be meaningless due to the differences in methodology used.
Within the activity
shown by drimane derivatives against the most sensitive fungus (E. floccosum),
the following conclusions can be extracted a) polygodial showed the best
activies with MIC = 3 µg/ml; b) when a bulky substituent (p-methoxy or p-hydroxycinnamoyl)
is present in position 1 of the polygodial, the activity diminishes 8 times
[compare MIC of (1) (= 3 µg/ml) with those of
compounds (2) and (4) (= 25 µg/ml each)]. This reduction seemed to be not dependent
on the substituent R1 on the benzene ring of the cinnamoyl moiety; c) the
presence of an OH in position 5 led to an inactive compound [compound (4),
MIC>100 µg/ml].
Considering that
the antifungal activity of drimane-type sesquiterpene dialdehydes was
previously attributed to their alfa, beta unsaturated aldehyde moiety (21), it
is interesting to note that drimanial 3, which possesses this structural
feature, did not display any activity up to 100 µg/ml.
Within the activity
of cinnamoyl esters 2, 3 and 4, it is interesting to note that
the change of a methoxyl by a hydroxyl group on the benzene ring (2 →
4) does not modify the antifungal behavior of these compounds (MIC = 25 µg/ml
against E. floccosum for both compounds) while the presence of an
hydroxyl group in the 5-position (2 → 3) led to complete
loss of activity.
CONCLUSIONS
Polygodial 1 together
with three other cinnamoyl derivatives 2-4 were isolated from the
chloroform extract of the bark of Drymis brasiliensis. Three of them
showed interesting antifungal activities, this being the first report on the
antifungal properties of cinnamoyl derivatives of polygodial.
Regarding antifungal activity of the isolated compounds, compound 1 strongly
inhibits E. floccosum with MIC values
of 3 µg/ml and compounds 2 and 3 inhibit the same fungus at higher concentrations (25 µg/ml), showing
that the three compounds contribute to the antifungal activity observed in the
whole bark. Although with a lesser incidence as the ethiological cause of
dermatophytoses, E. floccosum
produces arthroconidia, which survive for a longer time than other
dermatophytes, therefore constituting an environmental source of contagion,
sometimes leading to recurrent outbreaks of dermatophytosis in individuals and
in institutions (6). Although it is desirable to develop compounds having a
broad spectrum of activity, it is also important to keep in mind that the treatment of chronic
different tineas with the same broad spectrum antifungal agent leads sometimes
to a high resistance to the available antifungal agents (4). Thus, one of the strategies for overcoming
this problem is the treatment of fungal infections with the appropriate narrow
spectrum agent when the ethiological
agent is known (6).
Therfore, the selective
antifungal activity reported in this paper for drimanes isolated from D. brasiliensis opens the possibility that polygodial (1) or
its other drimane components (2) and (4) could be helpful for the
developing of new antifungal agents for treating dermatomycoses produced by E. floccosum,
usually very difficult to eradicate.
ACKNOWLEDGEMENTS
SAZ is grateful to Agencia de Promociones
Científicas y Tecnológicas de la Argentina (ANPyCT) PICT Redes 00260 and OEA
for financial support. OR and MVC acknowledge CONICET. This research is part
of Project X.7-PIBEAFUN (Iberian-American Project on Search and Development of
Antifungal Agents) of the Iberian-American Program for the Development of
Science and Technology (CYTED), sub-program X. VCF and RAY thank to CNPq/Brazil
for financial support.
REFERENCES
(1) Wong B, Klei B, Kozel T.
Inmunologic approaches and metabolite detection. The second NIAID Workshop in
Medical Micology, University of Arizona, Northern Arizona University,
Flagstaff, AZ, June 8-11, 1994.
(2) Ablordeppey S, Fan P, Ablordeppey JH,
Mardenborough L. Systemic antifungal agents against AIDS-related oportunistic
infections: current status and emerging drugs in development. Curr. Med. Chem.
1999; 6: 1151-95.
(3) Weitzman I, Summerbell, RC. The dermatophytes.
Clin. Microbiol.
Rev. 1995;
8: 240-59.
(4) Ghannoum MA, Rice, LB.
Antifungal agents: mode of action, mechanisms of resistance and correlation of
these mechanisms with bacterial resistance. Clin. Microbiol. Rev. 1999; 12: 501-17.
(5) White
T, Marr K, Bowden R. Clinical, cellular and molecular factors that contribute
to antifungal drug resistance. Clin. Microbiol. Rev. 1998; 11: 382-02.
(6)
Di Domenico B. Novel antifungal drugs. Curr.
Opin. Microbiol. 1999; 2: 509-15.
(7) Barrett D. From natural products to clinically
useful antifungals, Biochim. Biophys. Acta 2002: 1587:224-33.
(8) Cechinel Filho V, Schlemper V, Santos ARS,
Pinheiro TR, Yunes RA, Mendes GL, Calixto JB, Delle Monache F. Isolation and
identification of active compounds from Drimys
winteri barks. J. Ethnopharmacol. 1998; 62: 223-27.
(9) Malheiros A, Cechinel Filho V, Schmitt CB,
Santos ARS, Calixto JB, Delle Monache F, Yunes RA. A sesquiterpene drimane with
antinociceptive activity from Drimys
winteri barks. Phytochemistry
2001; 57: 103-7.
(10) Cechinel Filho V, Malheiros A, Schmitt C, Yunes RA, López S,
Sortino M, Escalante A, Castelli MV, Roveri O, Zacchino, SA. Estudio del potencial antifúngico de
plantas medicinales brasileras. I Congreso Iberoamericano de Química Fina
Farmacéutica CYTED- Universidad de Salamanca, Poster number C-59, 2002, p 113.
(11)
Lee SH, Lee JR, Lunde C, Kubo I. In vitro antifungal susceptibilities of Candida albicans and other fungal
pathogens to polygodial, a sesquiterpene dialdehyde, Planta Med. 1999; 65:
204-8.
(12)
Wright L, Scott E, Gorman S. The sensitive of mycelium, arthrospores and
microconidia of Trichophyton
mentagrophytes to imidazoles determined by in vitro tests. J. Antimicrob. Chemother. 1983; 12: 317-23.
(13) Barnes C, Loder J. The structure of Polygodial a
new Sesquiterpene Dialdehyde from Polygonum
hydropiper. Aust. J. Chem. 1962;15:
322-327.
(14) Taniguchi M, Chapya A, Kubo I, Nakanishi K.
Screening of East African Plants for Antimicrobial activity. Chem. Pharm. Bull.
1978; 26: 2910-1913.
(15) Kubo I, Lee Y, Pettei M, Pilkewicz F, Nakanishi
K. Potent army worm antifeedants from the East African Warburgia plants. Chem. Comm. 1976; 24: 1013-1014.
(16) McCallion R, Cole A, Walker J, Blunt J, Munro M.
Antibiotic Compounds from New Zealand Plants, II: Polygodial, an anti-Candida agent from Pseudowintera colorata. Planta Medica 1982; 44: 134-138.
(17) de Almeida Alves T, Lacerda Ribeiro F, Kloos H,
Zani C. Polygodial, the fungitoxic component from the Brazilian medicinal plant
Polygonum punctatum, Mem., Inst. Oswaldo Cruz (Rio de
Janeiro). 2001; 96: 831-833.
(18) Taniguchi M, Yano Y, Tada E, Ikenishi K, Oi S,
Haraguchi H, Hashimoto K, Kubo I. Mode of action of polygodial an antifungal sesquiterpene
dialdehyde. Agric. Biol. Chem. 1988; 52: 1409-1414.
(19)
Lunde C, Kubo I, Effect of polygodial on the mitochondrial ATPase of Saccharomyces cerevisiae, Antimicrobial
Agents and Chemotherapy 2000; 44: 1943-1953.
(20) Lee S, Lee J, Lunde C, Kubo I. In vitro antifungal susceptibilities of Candida albicans and other fungal
pathogens to polygodial, a sesquiterpene dialdehyde. Planta Medica 1999; 65:
204-208.
(21)
Taniguchi M, Adachi T, Oi S, Kimura A, Katsumara S, Isoe S, Kubo I.
Structure-activity relationship of the Warburgia
sesquiterpene dialdehydes. Agric. Biol. Chem. 1984; 48: 73-78.