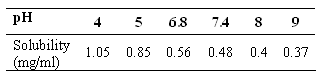J Pharm Pharmaceut Sci (www.cspscanada.org) 8(1):54-62, 2005
Transbuccal delivery of lamotrigine across porcine buccal mucosa: in vitro determination of routes of buccal transport.
Rajashree Mashru, Vijay Sutariya1, Mayur Sankalia, Jolly Sankalia
Pharmacy Department, Faculty of Technology and Engineering, The M. S. University of Baroda, Vadodara, IndiaReceived 4 October, 2004, Revised 6 December 2004, Accepted 12 January 2005, Published 28 February 2005
PDF Version
Abstract
PURPOSE: To determine the major routes of buccal transport of lamotrigine and to examine the effects of pH on drug permeation. METHODS. Transbuccal permeation of lamotrigine across porcine buccal mucosa was studied by using in-line Franz type diffusion cell at 37șC. The permeability of lamotrigine was determined at pH 4.0 to 9.0. The permeability of unionized (Pu) and ionized (Pi) species of drug were calculated by fitting the data to a mathematical model. Lamotrigine was quantified by using the HPLC method. RESULTS. The steady state flux increased linearly with increasing the donor concentration (r2 = 0.9639) at pH 7.4. The permeability coefficient and the partition coefficient of the drug increased with increasing the pH. The values of Pu and Pi were 0.7291 ∞ 10-5 cm/sec and 0.2500 ∞ 10-5 cm/sec, respectively. The observed permeability coefficients and the permeability coefficients calculated from mathematical model at various pH showed good linearity (r2 = 0.9267). The total permeability coefficient increased with increasing the fraction of unionized form of the drug. CONCLUSION. Lamotrigine permeated through buccal mucosa by a passive diffusion process. The partition coefficient and pH dependency of drug permeability indicated that lamotrigine was transported mainly via the transcellular route by a partition mechanism.
Introduction
The drug delivery through oral mucosa offers a number of advantages over oral delivery, especially for those drugs that have poor solubility, poor bioavailability, and/or those drugs that suffer from extensive first pass metabolism in the liver. Conceivably, buccal delivery systems may provide for easy administration with little or no irritation, thereby increasing patient compliance. In addition, gastric acid or digestive enzyme-mediated degradation in the gastrointestinal tract is also avoided. Moreover, absorption following oral mucosal administration is not influenced by the potential variation in the gastric-emptying rate or the presence of food (1-7).
Weak acids and weak bases are subjected to pH-dependent ionization. It is presumed that ionized species penetrate poorly through the oral mucosa compared with non-ionized species. An increase in the amount of non-ionized drug is likely to increase the permeability of the drug across an epithelial barrier, and this may be achieved by a change of pH of the drug delivery system. It has been reported that pH has effect on the buccal permeation of drug through oral mucosa (8-15). Previous drug absorption studies have demonstrated that buccal absorption through oral mucosa for drugs such as morphine sulphate (8), nicotine (9, 10), flecainide (11), sotalol (11), propanolol (12) and others (13-15) changed with changing the pH.
The examination of penetration route for transbuccal drug delivery is important because it is fundamental to select the proper penetration enhancer to improve the drug permeability. Previous drug absorption studies have demonstrated that oral mucosal absorption of amines and acids at constant concentration is proportional to their partition coefficients (16, 17). Similar dependencies on partition coefficients were obtained for acyclovir (15), β -adrenoceptor blocking agents (18), tetramethylpyrazine (19), substituted acetanilide (20) and others (16, 17).
Based on the cellular structure of the oral mucosa, there are two possible pathways for passive drug transportation - the paracellular route and the transcellular route (21-23). The flux of drug through the membrane under sink condition for paracellular route can be written as equation 1 (15, 19, 24).
Where, Dp = diffusion coefficient of the permeate in the intercellular spaces, hp = pathlength of the paracellular route, ε = area fraction of the paracellular route and Cd = donor drug concentration.
The flux of drug through the membrane under sink condition for transcellular route can be written as equation 2 (15, 19, 24).
Where, Kc = partition coefficient between lipophilic cell membrane and the aqueous phase, Dc = diffusion coefficient of the drug in the transcellular spaces and hc= pathlength of the transcellular route.
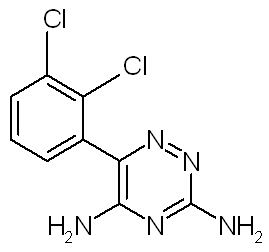
Lamotrigine, 6-(2,3-dichlorophenyl)-1,2,4-triazine-3,5-diamine is an antiepileptic agent used as a monotherapy and as an adjunct to treatment with other antiepileptic agents for partial seizures and primary and secondary generalized tonic-clonic seizures. It is also used for seizures associated with the Lennox-Gastant syndrome. It is extensively metabolized in the liver following oral administration (25). We consider that buccal administration of lamotrigine is one of the most attractive routes for systemic delivery when the bioavailability following oral administration is insufficient due to such effects as first pass hepatogastrointestinal metabolism. Therefore, the delivery of lamotrigine for systemic use via buccal mucosa was investigated. The molecular structure of lamotrigine is given in Figure 1. The aim of present study was to investigate the permeation of lamotrigine across porcine buccal mucosa ex vivo, the effect of the ionization state of lamotrigine at different pH on buccal permeation and to understand the in vitro transport of lamotrigine across porcine mucosa based on varying pH.
Figure 1: MOLECULAR STRUCTURE OF LAMOTRIGINE.
Materials and Methods
Materials
Lamotrigine was received as a gift sample from Torrent Pharmaceutical Ltd., Ahmedabad, India. Disodium hydrogen phosphate and citric acid were purchased from S. D. Fine Chemicals Ltd., Mumbai, India. HPLC grade methanol and acetonitrile were purchased from Loba Chemicals Ltd., Baroda, India. Other reagents used were of analytical grade and were used without additional purification.
Solubility Measurement
Solubility of lamotrigine was determined at several pH 4.0, 5.0, 6.8, 7.4, 8.0 and 9.0. Excess of lamotrigine was added to 10 ml of Mcllvaine buffer solutions at each level. The samples were stirred in a conical flask for 24 hour at 37ºC. The pH of the samples was checked, adjusting the pH with 0.1 M citric acid whenever necessary. The suspensions were filtered using a 0.45 micron whatman filter paper. The concentration of lamotrigine in the filtrate was determined spectrophotometrically by measuring absorbance at 305 nm (26).
Determination of Partition Coefficient
1-Octanol was used to represent the biomembrane. The partition coefficients between 1-octanol and Mcllvaine buffer solutions at different pH (from 4.0 to 9.0) at 37ºC were determined by shake-flask method (26). 1-Octanol and buffer solution were co-saturated with each other for 24 hr at 37ºC before use. 1-Octanol (5 ml) was shaken with 5 ml buffer solution containing lamotrigine (1 mg/ml) for 8 hrs at 37ºC. The mixture was then centrifuged at 2000 rpm for 10 minutes and the concentration of drug in each phase was determined spectrophotometrically by measuring absorbance at 305 nm in aqueous phase. The partition coefficient (Kp ) was calculated from the equation 3.
Where C1 is the original concentration of drug in aqueous phase and C2 is the final concentration of drug in aqueous phase.
Collection and Preparation of Buccal Tissue
Porcine buccal tissue was chosen, because its non-keratinized morphology is quite similar to human buccal epithelium (1, 11). Buccal tissue of guinea pig was removed after sacrificing the animal and was stored in Krebs buffer pH 7.4 and was immediately transported to the experimental set-up. The buccal mucosa membranes were separated by removing the underlying connective tissues using surgical scissors, making sure that the basal membrane was still present (27-29). The tissue slices with the thickness ranged from 500-600 mm were mounted between donor and receiver chambers of the diffusion cells for permeation studies.
In Vitro Permeation Study through Buccal Mucosa
The prepared buccal mucosa membranes with an approximate area of 4.00 cm2 were mounted between the donor and receiver chambers of Franz type diffusion cells with an available diffusion area of 1.76 cm2. The receiver chambers were filled with 10 ml of Mcllvaine buffer solution at pH 7.4 and the donor chambers were filled with 5 ml solution of lamotrigine of different concentration at Mcllvaine buffer pH 7.4 or solution of lamotrigine (0.4 mg/ml) in Mcllvaine buffer solutions of different pH (4.0, 5.0, 6.8, 7.4, 8.0, 9.0). Samples (0.3 ml) were withdrawn from receptor compartment at predetermined time interval (10, 30, 60, 90, 120, 150 and 180 minutes), replaced with the same volume of fresh medium and subsequently assayed by HPLC method. The amount of drug present in donor compartment was determined and was plotted as a function of time. The permeability coefficient (P) was calculated from the linear part of the curves as equation 4 (19).
Where, A = surface area of diffusion, dQ/dt = amount of drug permeated per unit time at steady state and Cd = donor drug concentration.
The permeability of lamotrigine was evaluated at different pH (from 4.0 to 9.0). The steady state flux (Jss = P ∞ Cd ) of the drug at pH 7.4 was calculated at different drug concentration. Permeability coefficients of unionized (Pu ) and ionized species (Pi ) of lamotrigine at different pH were also calculated.
Drug Retention in Mucosa
The mucosa was separated from the diffusion cell after the permeation run of donor solution containing 0.4 mg/ml lamotrigine at various pH and was homogenized using a mortar and pestle and extracted three times with 10 ml methanol. The organic layer was centrifuged at 7000 rpm for 10 minutes to separate the cellular components. The extract was diluted suitably and was analyzed by HPLC method.
HPLC Method
The HPLC system consisted of an LC 290 pump (Model LC 290, Perkin-Elmer, Inc., MA, USA) with UV detector (Model 290, Perkin Elmer, Inc., MA, USA) set at 305 nm, PE Nelson 1020 integrator and PE Nelson 1020 computer system. The samples were injected manually using a rheodyne injector (Model 7125, Rheodyne, Cotati, California, USA) with a 20-ìl loop. HPLC column (250 ∞ 4.6 mm) was a 5 micron C18 column (Hypersil, BDS, reverse phase) from Thermo Electron Corporation, USA. The mobile phase consisted of 0.01M phosphate buffer: methanol: acetonitrile (70:20:10) and was filtered through a 0.45-micron whatman filter paper before use. The chromatography was performed isocratically at a flow rate of 1.0 ml/min at room temperature (approximately 29 ºC) and retention time of 4.8 minutes was obtained. The amount of lamotrigine present in sample was calculated from prepared standard calibration curve having correlation coefficient value more than 0.9997. The calibration graph was linear in the concentration range of 0.1-1.5 mg/ml. The precision and accuracy of the method were between 0.94-2.68 % and 98.83-101.45 %, respectively.
Results
Effect of pH on Solubility
The solubility of lamotrigine at different pH buffer solution decreased with increasing pH. The results are shown in Table 1.
Table 1: Solubility of lamotrigine at different pH.
Permeation Parameters
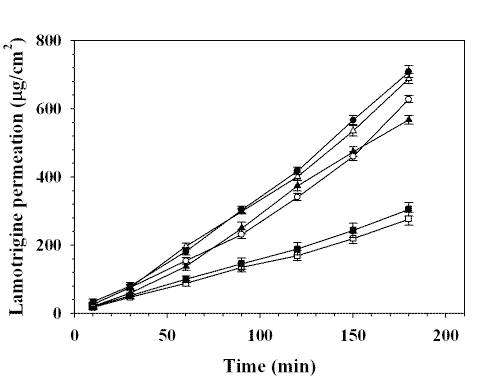
The permeation profiles of lamotrigine in Mcllvaine buffer solution (0.4 mg/ml) having different pH values are shown in Figure 2.
Figure 2: PERMEATION PROFILES OF LAMOTRIGINE THROUGH PORCINE BUCCAL MUCOSA: (❏) pH 4.0, (■) pH 5.0, (▲) pH 6.8, (❍) pH 7.4, (Δ) pH 8.0, (•) pH 9.0. EACH POINT REPRESENTS THE MEAN ± S. D. OF THREE EXPERIMENTS.
The permeated parameters such as steady state flux rate and permeability coefficient are given in Table 2. The permeated amount of drug increased linearly after 1 hr at each pH value.
Table 2: Permeation parameters of lamotrigine (donor compartment concentration - 0.4 mg/ml).
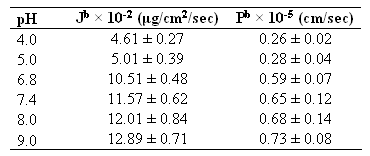
bThe values of steady state flux (J, mg/cm2/sec) and permeability coefficient (P, cm/sec) were calculated from the straight line obtained by plotting the permeated amount of drug (mg/cm2) versus time (sec). Each value represents the mean ± S. D. (n=3).
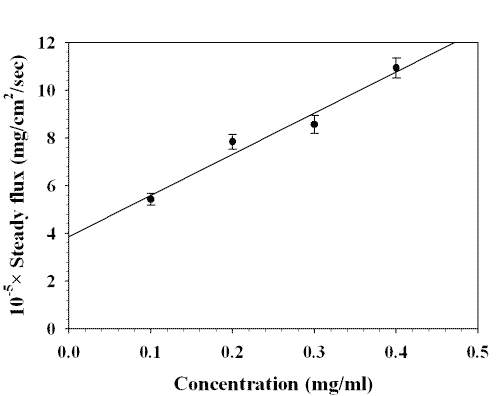
The steady state flux of lamotrigine at pH 7.4 increased with increasing the donor concentration. A linear relationship was observed between the flux and drug concentration (r2 =0.9639) which is shown in Figure 3.
Figure 3: EFFECT OF DONOR CONCENTRATION OF LAMOTRIGINE ON STEADY STATE FLUX AT pH 7.4. EACH POINT REPRESENTS THE MEAN ± S. D. OF THREE EXPERIMENTS.
The linear relationship between the steady state flux at pH 7.4 and donor drug concentration showed that the transport of drug through buccal mucosa at the concentration range from 0.1 to 0.4 mg/ml was a passive diffusion process.
Effect of pH on Partition Coefficient and Permeability
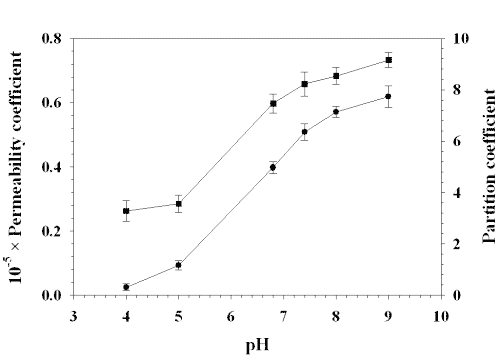
Both the apparent 1-octanol/buffer solution partition coefficient and the permeability coefficient increased with increasing the pH of buffer solution in donor chamber (Fig. 4).
Figure 4: EFFECT OF pH ON PERMEABILITY COEFFICIENT AND PARTITION COEFFICIENT OF LAMOTRIGINE: (■) PERMEABILITY COEFFICIENT, (•) PARTITION COEFFICIENT. EACH POINT REPRESENTS THE MEAN ± S. D. OF THREE EXPERIMENTS.
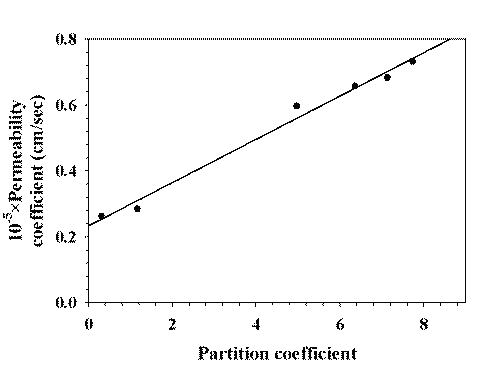
Excellent linearity (r2 = 0.9879) was observed between the permeability coefficient and the partition coefficient (Fig. 5).
Figure 5: CORRELATION OF 1-OCTANOL/BUFFER PARTITION COEFFICIENT AND PERMEABILITY COEFFICIENT.
According to equations mentioned above and the assumption that drug will have the same partition tendency at 1-octanol and the biomembrane, the permeability of drug should have nothing to do with the partition coefficient if it goes through the paracellular route (Eq. 1), while the permeability shall vary with the partition coefficient if the drug was transported via the transcellular route (Eq. 2). To further explain our experimental results, we assume that unionized form mainly goes through transcellular route and the ionized form transports via the paracellular route, the steady state flux can be expressed by the equation 5 (19).
Where,
Jt = total flux of drug, Ju = transcellular flux, Ji = paracellular flux, Cu = concentration of unionized species, Ci = concentration of ionized species, Ct = total drug concentration, Pt = total drug permeability.
So, the total rug permeability can be written as equation 6.
Lamotrigine has one dissociation constant and its value is 1.995 ∞ 10 -0.6 . It is a basic drug with pKa value of 5.7 (30). The percentage of different species at a given pH can be calculated by using the Henderson-Hesselbalch equation which is given as equation 7.
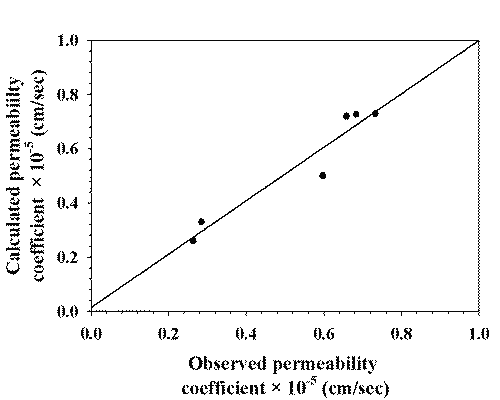
Pu and Pi were calculated by fitting Pt , Cu /Ct , and Ci /Ct at different pH to equation 6. The calculated value for Pu was 0.7291 ∞ 10-5 cm/sec which is about three times higher than Pi , 0.2500 ∞ 10-5 cm/sec. The calculated permeability coefficient (Pcal ) was plotted with the observed permeability coefficient at various pH values which is shown in Figure 6.
Figure 6: CORRELATION BETWEEN OBSERVED PERMEABILITY COEFFICIENT AND CALCULATED PERMEABILITY COEFFICIENT.
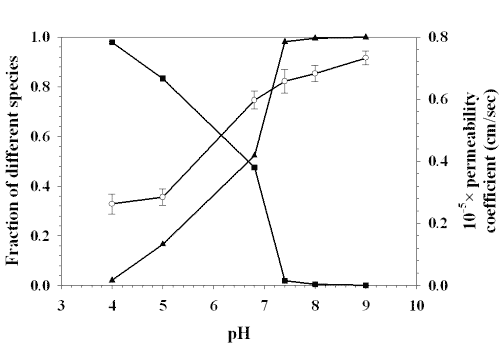
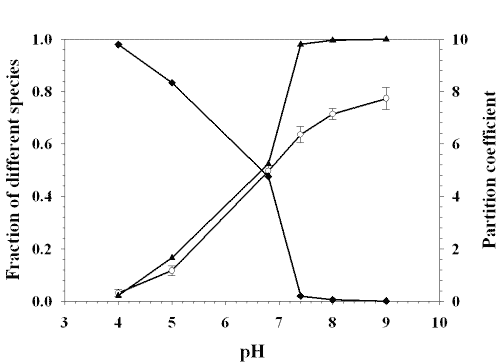
Figure 7 shows the relationship between the permeability coefficient and the fraction of different species of lamotrigine while figure 8 shows the relationship between the partition coefficient and the fraction of different species.
Figure 7: RELATIONSHIP BETWEEN PERMEABILITY COEFFICIENT AND THE FRACTION OF DIFFERENT SPECIES OF LAMOTRIGINE: (■) FRACTION OF IONIZED SPECIES, (❍) PERMEABILITY COEFFICIENT, (▲) FRACTION OF UNIONIZED SPECIES. EACH POINT REPRESENTS THE MEAN ± S. D. OF THREE EXPERIMENTS.
Figue 8: RELATIONSHIP BETWEEN PARTITION COEFFICIENT AND THE FRACTION OF DIFFERENT SPECIES OF LAMOTRIGINE: (■) FRACTION OF IONIZED SPECIES, (❍) PARTITION COEFFICIENT, (▲) FRACTION OF UNIONIZED SPECIES. EACH POINT REPRESENTS THE MEAN ± S. D. OF THREE EXPERIMENTS.
The partition coefficient and the total permeability coefficient of lamotrigine increased with increasing the fraction of unionized form.
Drug Content in Mucosa
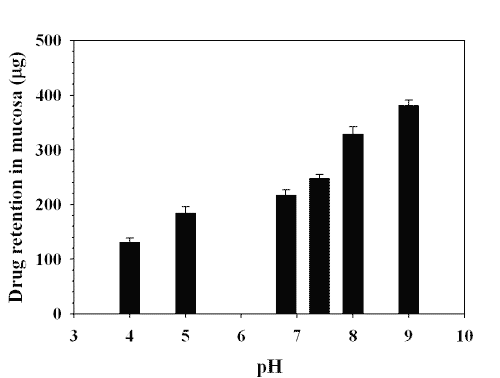
Results of drug remained in mucosa after run at various pH is shown in Figure 9.
Figure 9: CONTENT OF LAMOTRIGINE (mg) IN MUCOSA AFTER RUN AT VARIOUS pH. EACH POINT REPRESENTS THE MEAN ± S. D. OF THREE EXPERIMENTS.
The value of drug content in mucosa increased with increasing the pH which indicated that buccal permeation of lamotrigine increased with increasing the pH. The fraction of unionized species of drug increases with increasing the pH. The increase of drug content in mucosa with increasing the pH might be due to permeation of more unionized species at higher pH value through the transcellular route across buccal mucosa.
Discussion
Drugs ability to diffuse across the membranes is frequently expressed in terms of their lipid-water partition coefficient, which is a measure of the relative affinity of a drug for the lipid and aqueous phases. But it is not true in vivo model for the transbuccal diffusion of drug and also does not take into account of the influence of pH of body fluids and pKa of drug. The transmembrane diffusion process is passive in nature and depends on a concentration differential as the driving force, each molecule requiring kinetic energy to effect a net movement down this gradient. Permeant molecules must therefore diffuse through the vehicle in which they are contained to the mucosal interface and have to partition from the vehicle into the upper layers of the tissue. From here molecules must diffuse within the mucosa, equilibrating laterally, and must emerge, eventually under steady-state conditions, from the distal surface of the tissue. Adsorptive interaction might be extensive in this layer, forming a reservoir of the permeant molecules. Further partitioning into neighboring tissue strata or into the receptor fluid then takes place under the influence of the concentration gradient, and adsorption might occur once again. Initially the concentration gradient across the mucosa will not be linear as the permeant equilibrates within the tissue. However, after sufficient time has elapsed, steady state will be achieved and the effective permeant concentration at all points in the tissue will remain constant.
Penetration of drug into tissues is dependent on the ionization state of the drug molecule. At lower pH values of the buffer system used in this study, i.e., 4.0, approximately 98.05 % of the lamotrigine is present in their dissociated forms. Although this improves diffusion through the hydrophilic outer layers of the epithelium, it does not facilitate penetration of the lipoidal layer, which is thought to constitute the major permeability barrier of buccal mucosa (31, 32). At higher pH value, i.e., 9.0, approximately 5.01 % of the lamotrigine is present in their dissociated forms which improve diffusion through lipoidal layer. It is clear that the flux rates across buccal mucosa at pH 9.0 are statistically significantly higher than those of pH 4.0 (Fig. 2). This is also reflected in the permeability coefficients, showing the higher permeation of lamotrigine at higher pH than that of lower pH. The permeability coefficient increased as the pH value increased because the solubility of drug decreased with increasing the pH. Good linearity (r2 = 0.9267) was observed between the observed permeability coefficient and the calculated permeability coefficient at various pH which indicates the reliability of the mathematical model used for the calculation of Pi and Pu .
According to the pH-partition hypothesis, only the nonionized form of the drug is able to cross lipodal membranes in significant amount. The effect of pH on drug absorption for ionizable compound has been extensively studied (15, 33-35). If a single species of drug is transported via the paracellular route (Eq.1), the permeability of the drug should be independent of partition coefficient. As shown in figure 4, this is not the case for lamotrigine. Conversely, if the drug is transported via the transcellular route (Eq.2), the permeability of the drug should vary with the partition coefficient. Thus, the pH dependence of the permeation of ionizable drug actually reflects the drug penetration route, because the partition coefficient of ionizable drug is pH dependent. If a drug is transported via the transcellular route, the drug absorption rate is also pH dependent. While in vitro permeation of lamotrigine varies with pH, the variation of the permeability coefficients of lamotrigine with pH far exceeds that of the partition coefficients. Therefore, a model of permeation of a single species would not be consistent with the data (Fig. 4).
The calculated value of permeability coefficient of unionized species was about three times higher than ionized species. In our study, the permeabilities of drug was proportional to the partition coefficients (Fig. 5) and increased with increasing the pH (Fig. 4), which showed a transcellular route to be the main pathway for the buccal permeation of the drug. The transport pathway of drug depends on the lipophilicity of the drug. Hydrophilic compounds mainly cross the buccal mucosa via the paracellular route while the transcellular pathway is main transport route for lipophilic compounds (11). Lamotrigine is a lypophilic drug with a molecular weight of 265.1. Therefore, the transcellular route is most likely the dominant pathway. A maximum partition coefficient (1-octanol/water) of 7.74 was found at pH 9.0, while the partition coefficient at pH 4.0 was 0.31. The increased lipophilicity at pH 9.0 as compared with pH 4.0 resulted in a statistically significant increase in permeation across porcine buccal mucosa. The increased lipophilicity made the transcellular pathway to play a significant role in the permeation of lamotrigine.
Conclusions
Permeability coefficient and steady state flux of lamotrigine across porcine buccal mucosa increased with increasing the pH values. The 1-octanol/buffer partition coefficient of the drug also increased with increasing the pH. The permeability coefficient of unionized species was greater than that of ionized species which indicated that the unionized species of lamotrigine penetrated well through porcine buccal mucosa and the permeation was a function of pH. It is concluded that main transport route of buccal permeation of lamotrigine is through transcellular route.
References
Gandhi, R.B. and Robinson, J.R., Oral cavity as a site for bioadhesive drug delivery. Adv Drug Deli Rev, 13:43-74, 1994.
Patterson, K.W. and Keane, P.W., Use of the buccal route for the administration of an antiemetic. Pharm Res, 6:160-166, 1989.
Rathbone, M.J. and Hasgraft, J., Absorption of drugs from the human oral cavity. Int J Pharm, 71:9-24, 1991.
Khanvilkar, K., Donovan, M.D. and Flanagan, D.R., Drug transfer through mucus. Adv Drug Del Rev, 48:173-193, 2001.
Senel, S. and Hincal, A.A., Drug permeation enhancement via buccal route: possibilities and limitations. J Contr Rel, 72:133-144, 2001.
Junginger, H.E., Hoogstraate, J.A. and Verhoef, J.C., Recent advances in buccal drug delivery and absorption—in vitro and in vivo studies. J Contr Rel, 62:149-159, 1999.
Chen, L.L., Chetty, D.J. and Chien, Y.W., A mechanistic analysis to characterize oramucosal permeation properties. Int J Pharm, 184:63-72, 1999.
Al-sayed-omar, O., Johnson, A. and Turner, P., Influence of pH on the buccal absorption of morphine sulphate and its major metabolite, morphine-3-glucuronide. J Pharm Pharmcol, 39:934-935, 1987.
Nair, M.K., Chetty, D.J., Ho, H. and Chein, Y.W., Biomembrane permeation of nicotine: mechanistic studies with porcine mucosa and skin. J Pharm Sci, 86:257-262, 1997.
Nielson, M.N. and Rassing, M.R., Nicotine permeability across the buccal TR 146 cell culture model and porcine mucosa in vitro: effect of pH and concentration. Eur J Pharm Sci, 16:151-157, 2002.
Deneer, V.H., Drese, G.B., Roemele, P.E., Verhoef, J.C., Lie-A-Huen, L., Kingma, J.H., Brouwers, J.R. and Junginger, H.E., Buccal transport of flecainide and sotalol: effect of a bile salt and ionization state. Int J Pharm, 241:127-134, 2002.
Coutel-Egros, A., Maitani, Y., Veillard, M., Machida, Y. and Nagai, T., Combined effects of pH, cosolvent and penetration enhancers on the in vitro buccal absorption of propanolol through excised hamster cheek pouch. Int J Pharm, 84:117-128, 1992.
Nielsen, H.M. and Rassing, M.R., TR146 cells grown on filters as a model of human buccal epithelium: III. Permeability enhancement by different pH value, different osmolarity value, and bile salts. Int J Pharm, 185:215-225, 1999.
Gandhi, R.B. and Robinson, J.R., Mechanisms of penetration enhancement for transbuccal delivery of salicylic acid. Int J Pharm, 85:129-140, 1992.
Shojaei, A.H., Berner, B. and Li, X., Transbuccal delivery of acyclovir: I. In vitro determination of buccal transport. Pharm Res, 15:1182-1188, 1998.
Beckett, A.H. and Moffat, A.C., Correlation of partition coefficients in n-heptane –aqueous systems with buccal absorption data for a series of amines and acids. J Pharm Pharmcol, 21: 144S-150S, Suppl, 1969.
Beckett, A.H. and Triggs, E.J. Buccal absorption of basic drugs and its application as an in vivo model passive drug transfer through lipid membrane. J Pharm Pharmcol, 19:31S-41S, Suppl, 1967.
Le Brun, P.P.H., Fox, P.L.A., De Vries, M.E. and Bodde, H.E., In vitro penetration of some β-adrenoreceptor blocking drugs through porcine buccal mucosa. Int J Pharm, 49:141-145, 1989.
Chen, L., Hui-Nan, X. and Xiao-Ling, L. In vitro permeation of tetramethylpyrazine across porcine buccal mucosa. Acta Pharmacol Sin, 23:792-796, 2002.
Garren, K.W. and Repta, A.J., Buccal drug absorption-II. In vitro diffusion across the hamster cheek pouch. J Pharm Sci, 78:160-164, 1989.
Chettu, D.J., Chen, L.L. and Chien, Y.W., Characterization of captopril sublingual permeation: determination of routes and mechanisms. J Pharm Sci, 90:1868-1877, 2001.
Ishizawa, T., Hayashi, M. and Awazu, S., Paracellular and transcellular permeabilities of fosfomycin across small intestinal membrane of rat and rabbit by voltage-clamp method. J Pharmacobiodyn, 14:583-589, 1991.
Toropainen, E., Ranta, V., Vellonen, K., Palmgren, J., Tavitie, A., Laavola, M., Suhonen, P., Hamalanen, K.M., Auriola, S. and Urtti, A., Paracellular and passive transcellular permeability in immortalized human corneal epithelial cell culture model. Eur J Pharm Sci, 20:99-106, 2003.
Zhang, H. and Robinson, J.R., In Vitro Methods for Measuring Permeability of the Oral Mucosa, in Swarbrick, J: Boylan, JC, (eds), Oral Mucosal Drug Delivery. 1st ed., Vol 74, Marcel Dekker, INC, New York, NY, pp 85-100, 1996.
Brodie, M.J., Lamotrigine. Lancet, 339:1397-1400, 1992.
Takahashi, K., Sakano, H., Rytting, H.J., Numata, N. and Kuroda, S., Influence of pH on the permeability of p- toluidine and aminopyrine through shed snake skin as a model membrane. Drug Dev Ind Pharm, 27:159-164, 2001.
Hoogstraate, A.J., Senel, S., Cellander, C., Verhoef, J.C., Junginger, H.E. and Bodde, H.E., Effects of bile salts on transport routes and routes of FITC-lebelled compounds across porcine buccal epithelium in vitro. J Contr Rel, 40:211-221, 1996.
Tsutsumi, K., Obata, Y., Nagai, T., Loftsson, T. and Takayama, K., Buccal absorption of ergotamine tartrate using the bioadhesive tablet system in guinea pigs. Int J Pharm, 238:161-170, 2002.
Cui, Z., and Mumper, R.J., Buccal transmucosal delivery of calcitonin in rabbits using thin-film composite. Anesth Analg, 74:937-938, 1992.
Maryadele, J.O.; Ann, S. and Patricia, E.H., The Merck Index, 13th edition, Merck and Co, Inc, New Jersey, pp 5371, 2001.
Squier, C.A., The permeability of oral mucosa. Crit Rev Oral Biol Med, 2:13–32, 1991.
Pieter, V.D.B., Armorel, D.V.E., Heiner, I.S., Ianda, V. and Marli, J., Transmucosal permeation of topically applied diclofenac and piroxicam. The J Appl Res, 3:505-511, 2003.
Al-Sayed-Omar, O., Johnson, A. and Tunder, P., Influence of pH on the buccal absorption of morphine sulphate and its major metabolite, morphine-3-glucoronide. J Pharm Pharmcol, 39:934-935, 1987.
Barsuhn, C.L., Olanoff, D.D., Gleason, E.L., Adkins, E.L. and Ho, N.F.H., Human buccal absorption of flubriprofen. Clin Pharmacol Ther, 44:225-231, 1998.
Hick, D., The buccal absorption of some β-adrenoceptor blocking drugs. Br J Pharmacol, 47:680-681, 1973.
Corresponding Author: Vijay Sutariya, Pharmacy Department, Faculty of Technology and Engineering, The M. S. University of Baroda, Kalabhavan, Vadodara, India, vsutariya@yahoo.co.in
Published by the Canadian Society for Pharmaceutical Sciences.
Copyright © 1998 by the Canadian Society for Pharmaceutical Sciences.
http://www.cspscanada.org