J Pharm Pharmaceut Sci (www.cspscanada.org) 8(1):115-123, 2005
Protective role of tetrahydrocurcumin (THC) an active principle of turmeric on chloroquine induced hepatotoxicity in rats.
Leelavinothan Pari1, D. Rosalin Amali
Department of Biochemistry, Faculty of Science, Annamalai University, Annamalai Nagar, Tamil Nadu, IndiaReceived 10 December 2004, Revised 18 January 2005, Accepted 3 February 2005, Published 30 April 2005
PDF Version
Abstract
PURPOSE. Tetrahydrocurcumin (THC) is an antioxidative substance, which is derived from curcumin, the component of turmeric. In the present investigation, the effect of THC and curcumin against chloroquine (CQ) induced hepatotoxicity were studied in female Wistar rats. METHODS On single oral administration of CQ (970 mg/kg body weight) the activities of serum marker enzymes namely aspartate transaminase, alanine transaminase and alkaline phosphatase and the levels of bilirubin were significantly increased with significant alterations of lipids in serum and lipidperoxidation markers such as thiobarbituric acid reactive substances (TBARS) and hydroperoxides in plasma and liver were also elevated in CQ treated rats. The levels of non-enzymic antioxidants (vitamin C, vitamin E and reduced glutathione) and enzymic antioxidants (superoxide dismutase, catalase and glutathione peroxidase) were also decreased in CQ treated rats. Administration of THC (80 mg/kg body weight) and curcumin (80 mg/kg body weight) for 8 days before and 7 days after single administration of CQ significantly decreased the activities of serum markers and lipids in serum. In addition, the level of TBARS and hydroperoxides were significantly decreased with significant increase in non-enzymic and enzymic antioxidants on treatment with THC and curcumin. The biochemical observation was supplemented by histopathological examination of liver section. The results of the study reveal that THC shows more pronounced protective effect than curcumin against CQ induced toxicity.
Introduction
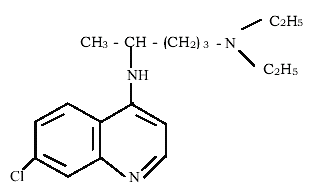
The synthetic quinoline drug-Chloroquine (CQ) (Figure 1) is commonly used in treatment of malaria. CQ is used for the treatment of a wide range of disease such as inflammation, extra intestinal amebiasis or gout [1] and rheumatoid arthritis [2].
Figure 1: The Structure of Chloroquine (C18H26CIN3).
Clinical treatment with CQ is often accompanied by serious side effects such as gastrointestinal upset, pruritus, headache, visual disturbances and cardiotoxic action [3]. CQ has been reported to cause liver damage [4, 5] and a severe life threatening toxic hepatitis at higher dosage [6]. CQ acts directly or indirectly and alters antioxidant status that makes certain organs, more susceptible to oxidative stress. Several studies have shown that CQ causes increased lipid peroxidation and decreased enzymic and non-enzymic antioxidants [7, 8].
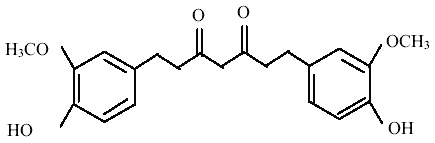
THC (Figure 2) is one of the major colourless metabolite of antioxidant action [9] obtained by the hydrogenation of curcumin, the biologically active principle of Curcuma longa (Turmeric), a common spices widely used in India [10].
Figure 2: Structure of tetrahydrocurcumin.
THC has possessed strong antioxidant action among all the curcuminoids [9, 11].
The antioxidant role of THC has been implicated in atherosclerotic lesion [12], anti inflammatory activity [9] and cancer [13].
Pari and Murugan [14] have reported the hepatoprotective effect of THC against erythromycin estolate induced toxicity. There is no other report available in the literature on the protective effect of THC and curcumin against CQ induced hepatic damage. The present study was designed to induce hepatotoxicity by CQ and to demonstrate the protective effect of THC in rats treated with chloroquine.
Materials and Methods
Chemicals
THC was a gift provided by Sabinsa Corporation, USA. Chloroquine was purchased from Ipca Laboratories Ltd., Ratlam, India and curcumin from the Daiwa Kasei Co., Ltd., Saitama, Japan. Thiobarbituric acid (TBA), butylated hydroxytoluene (BHT), reduced glutathione (GSH), 2,2-dipyridyl, xylenol orange, aspartic acid, alanine, a-ketoglutarate (a-KG), 2,4-dinitro phenyl hydrazine (DNPH), 5,5'-dithiobis-2-nitrobenzoic acid (DTNB) were obtained from Sigma chemical co (St. Louis, MO-US). The rest of the chemical utilized were obtained from local firm (India) and were of highest purity grade.
Animals
Female Wistar rats (180-200g) bred in the Central Animal House, Rajah Muthiah Medical College (RMMC), Annamalai University were used in the present study were maintained in accordance with the guidelines of the National Institute of Nutrition, Indian Council of Medical Research, Hydreabad, India and the study was approved by the ethical committee, Annamalai University.
Experimental Design
Animals were radomized and divided into 6 groups (n=6 in each group). Animals in Group I (untreated control), Group II, III, normal animals received curcumin (80mg/kg body weight) [15] and THC (80mg/kg body weight) [14], respectively in aqueous suspension daily using intragastric tube for 15 days. Group IV animals received single oral administration of CQ (970 mg/kg body weight) [5]. Animals from group V received CQ and curcumin (80 mg/kg body weight) for 8 days orally before the single oral administration of CQ and treatment with curcumin followed for 7 more days by intragastric intubation. Group VI received CQ and THC (80 mg/kg body weight) for 8 days orally before the single oral administration of CQ and treatment with THC followed for 7 more days by intragastric intubation.
Biochemical Analysis
After the experimental period, animals in different groups were sacrificed. Blood was collected in two different tubes i.e. one with anticoagulant, for plasma separation and another without anticoagulant to separate serum for various biochemical estimations. The liver was dissected out, washed in ice-cold saline, blotted dry, and weighed. Then homogenate was prepared in phosphate buffer 0.1M, pH 7.4 and used for the biochemical analysis.
Serum hepatospecific markers
Activities of serum Aspartate transaminase (AST) and Alanine transaminase (ALT) were assayed by the method of Reitman and Frankel [16]. 0.2 ml of serum with 1 ml of substrate (aspartate and a-ketoglutarate for AST; alanine and a-keto glutarate for ALT, in phosphate buffer pH 7.4) was incubated for an hour in case of AST and 30 minutes for ALT. 1 ml of DNPH solution was added to arrest the reaction and kept for 20 min in room temperature. After incubation 1 ml of 0.4N NaOH was added and absorbance was read at 540 nm. Activities expressed as IU/L.
Based on the method of King and Armstrong [17] alkaline phosphatase activity was assayed using disodium phenyl phosphate as substrate. The colour developed read at 680 nm after 10 min. Activities of ALP expressed as IU/L. Serum total bilirubin level was estimated based on Van den Berg reaction [18]. Diazotised sulphonilic acid (0.5 ml) reacts with bilirubin in diluted serum (0.2 ml serum + 1.8 ml distilled water) and forms purple coloured azobilirubin, which was measured at 540 nm.
Estimation of serum lipids
Extraction of lipids from serum carried out according to the procedure of Folch et al., [19] by using chloroform-methanol (2:1 V/V) mixture. From this total cholesterol [20], triglycerides [21], free fatty acids [22] and phospholipids [23] were estimated.
Estimation of lipid peroxidation
Lipid peroxidation in plasma and liver was estimated colorimetrically by measuring thiobarbituric acid reactive substances (TBARS) and hydroperoxides by the method of Nichans and Samuelson [24] and Jiang et al., [25], respectively. In brief, 0.1 ml of plasma was treated with 2 ml of (1:1:1 ratio) TBA - TCA - HCl reagent (TBA 0.37%, 0.25 N HCl and 15% TCA) and placed in water bath for 15 min, cooled and centrifuged and then clear supernatant was measured at 535 nm against reference blank.
Hydroperoxides was expressed as mM/dl. 0.1 ml of plasma was treated with 0.9 ml of Fox reagent (88 mg BHT, 7.6 mg xylenol orange and 0.8 mg ammonium Iron sulphate were added to 90 ml of methanol and 10ml of 250 mM sulphuric acid) and incubated at 37°C for 30 min. The colour developed was read at 560 nm.
Determination of non-enzymic antioxidants
Reduced glutathione (GSH) was determined by the method of Ellman [26]. 1 ml of supernatant (0.5 ml plasma /0.5ml liver homogenate precipitated by 2ml of 5% TCA) was taken and 0.5 ml of Ellman's reagent (0.0198% DTNB in 1% sodium citrate) and 3 ml of phosphate buffer (pH 8.0) were added. The colour developed was read at 412 nm.
Ascorbic acid (vitamin C) concentration was measured by Omaye et al., [27] method. To 0.5 ml of plasma/0.5ml liver homogenate 1.5 ml of 6% TCA was added and centrifuged (3500 g, 20 min). To 0.5 ml of supernatant, 0.5 ml of DNPH reagent (2% DNPH and 4% thiourea in 9N sulphuric acid) was added and incubated for 3 hours at room temperature. After incubation 2.5 ml of 85% sulphuric acid was added and colour developed was read at 530 nm after 30 min.
Vitamin E was estimated by the method of Desai [28]. Vitamin E was extracted from plasma/liver homogenate by addition of 1.6 ml ethanol and 2.0 ml petroleum ether to 0.5 ml plasma and centrifuged. The supernatant was separated and evaporated. To the residue, 0.2 ml of 0.2% 2, 2' - dipyridyl, 0.2 ml of 0.5% Ferric chloride was added and kept in dark for 5 min. An intense red colored layer obtained on addition of 4 ml butanol was read at 520 nm.
Assay of enzymic antioxidants
Superoxide dismutase (SOD) activity was determined by the modified method of NADH - Phenazinemethosulphate - nitroblue tetrazolium formazon inhibition reaction spectrophotometrically at 560 nm [29]. A single unit of enzyme was expressed as 50% inhibition of NBT (Nitroblue tetrazolium) reduction/min/mg protein. Catalase (CAT) was assayed colorimetrically as described by Sinha [30] using dichromate-acetic acid reagent (5% potassium dichromate and glacial acetic acid were mixed in 1:3 ratio). The intensity was measured at 620 nm and the amount of hydrogen peroxide hydrolyzed was calculated for the catalase activity.
Glutathione peroxidase (GPx) activity was measured by the method described by Rotruck et al., [31]. The activity was expressed based on inhibition of GSH. Protein was determined by the method of Lowry et al. [32] using Bovine Serum Albumin (BSA) as standard, at 660 nm.
Histopathological study
Small pieces of liver tissues were collected in 10% formalin for proper fixation. These tissues were processed and embedded in paraffin wax. Sections of 5-6μm in thickness were cut and stained with hematoxylin and eosin.
Statistics
Statistical evaluation of data was performed by using one-way analysis of variance (ANOVA) followed by Duncan's multiple range test (DMRT) [33].
Result
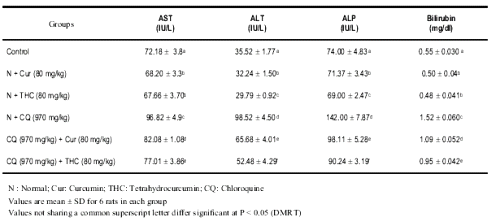
The changes in the activities of AST, ALT, ALP and level of bilirubin in normal and experimental animals are shown in Table. 1. The increased activities of AST, ALT, ALP and levels of bilirubin in serum due to CQ challenge were significantly decreased on treatment with THC and curcumin.
Table 1: Changes in the activities of serum AST, ALT, ALP and bilirubin in control and experimental rats.
The changes in the levels of serum lipids in control and experimental rats are illustrated in Table 2.
Table 2: Changes in the levels of serum cholesterol, triglycerides, free fatty acids and phospholipids in control and experimental rats.
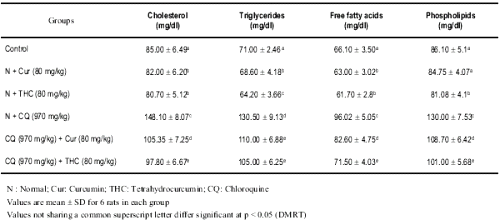
The levels of serum cholesterol, triglycerides, free fatty acids and phospholipids were highly altered in CQ treated rats when compared with control or curcumin/THC alone treated rats. THC treated rats offered a significant protection against CQ induced alteration in the serum lipids. THC was highly effective when compared with curcumin.
Table 3 depicts the levels of plasma and liver lipid peroxidation products such as TBARS and hydroperoxides in control and experimental animals.
Table 3: Changes in the levels of TBARS, hydroperoxides in plasma and liver of control and experimental rats.
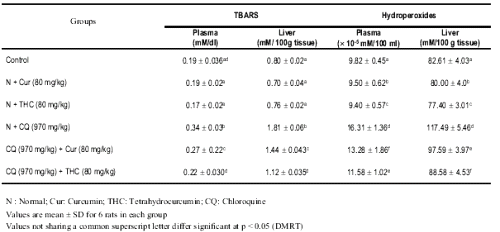
The levels of TBARS and hydroperoxides were considerably increased in rats treated with CQ as compared to curcumin and THC alone treated rats. Treatment with THC and curcumin resulted in a significant decrease in the levels of lipid peroxidation products. THC showed a better effect than curcumin.
The levels of plasma and liver vitamin C, vitamin E and reduced glutathione in control and experimental rats are presented in Table 4.
Table 4: Changes in the levels of plasma vitamin C, vitamin E and GSH in control and experimental rats.
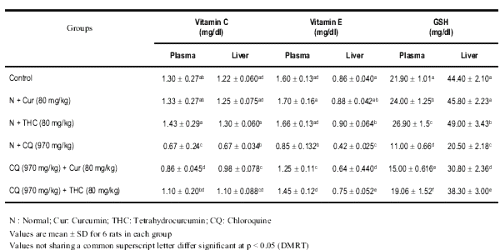
The levels of non-enzymic antioxidant were significantly depleted in rats treated with CQ. Treatment with THC or curcumin significantly increased the levels of non-enzymic antioxidants in CQ treated rats. The results of present study showed that THC has profound effect than curcumin.
The changes in the activities of enzymic antioxidants namely SOD, CAT, and GPx in liver of control and experimental animals are shown in Table 5.
Table 5: Changes in the activities of liver SOD, CAT, GPx in control and experimental rats.
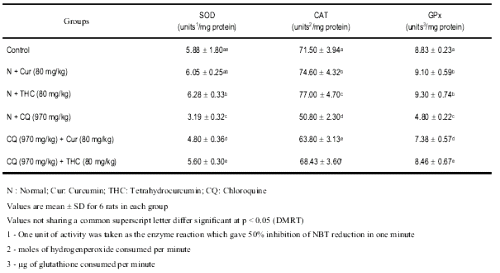
A significant decrease in the activities of enzymic antioxidants was noted after single administration of CQ. Upon administration of THC, the activities of enzymic antioxidants were significantly reversed to near normal.
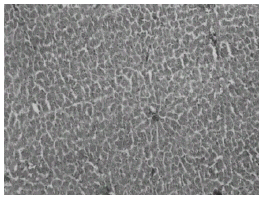
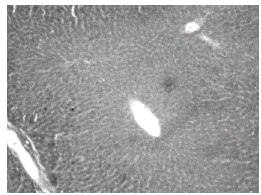
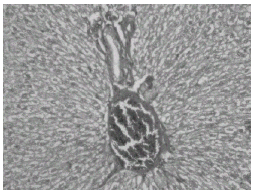
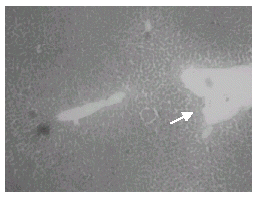
Histopathological studies showed that treatment with CQ caused liver damage including feathery degeneration and/microvesicular type of fatty generation (Fig. 5) sinusoidal dilatation and focal necrosis (Fig. 6) as compared with control liver (Fig. 3).
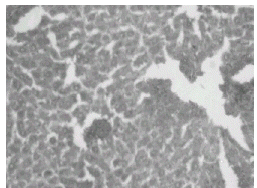
The above changes were reduced in liver of rats treated with THC (Fig. 7) as well as curcumin (Fig. 8).
Normal rats treated with THC showed near normal appearance of the liver (Fig. 4).
The effect of THC was more prominent than that of curcumin.
Figure 3: Control liver: H&E × 20.
Figure 4: Normal + tetrahydrocurcumin: H& E × 20 Normal appearance of liver.
Figure 5: Normal + Chloroquine-treated rat liver: H&E × 20. Hepatocytes show feathery degeneration and/ microvesicular type of fatty generation.
Figure 6: Normal + Chloroquine toxicity treated rat liver: H&E × 20. Sinusoidal dilatation and focal necrosis.
Figure 7: Chloroquine + curcumin-treated rat liver: H&E × 20. Normal hepatocytes with focal necrosis.
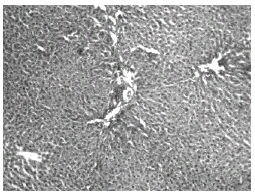
Figure 8: Chloroquine + Tetrahydrocurcumin-treated rat liver: H&E × 20. Normal hepatocytes with mild portal inflammation and normal hepatocytes.
Discussion
Serum AST, ALT, ALP and bilirubin are the most sensitive markers employed in the diagnosis of hepatic damage because these are cytoplasmic in location and are released into the circulation after cellular damage [34]. The increased activities of AST, ALT, ALP and the level of bilirubin in serum manifested the chloroquine induced hepatocellular damage. This was confirmed by our earlier study [4], in which the marked elevations of hepatic markers were reported. The other antimalarials like amodiaquine [35] and quinine [36] were also reported to induce hepatic damage.
Treatment with THC and curcumin significantly decreased the activities of AST, ALT, ALP and the level of bilirubin in serum suggesting that they offer protection by preserving the structural integrity of hepatocellular membrane against CQ. The above findings could be correlated with previous study, which reported that treatment with THC [14] significantly reduced the activities of serum markers in erythromycin-induced hepatotoxicity.
Farombi & Emerole [37] reported that CQ increased the cholesterol / phospholipid ratio, thereby disrupting membrane fluidity and leading to membrane alteration of function. In agreement with this, in our study also the levels of serum lipids increased in CQ treated rats suggesting that CQ has devastating effect on membrane stability and functional state. The similar result was observed in our previous study [4]. Administration of THC and curcumin showed protection against serum lipid changes caused by CQ, denoting a broad spectrum of hepatoprotective property. It was well correlated with our previous study [14], which showed that administration of THC significantly protected the erythromycin induced lipid changes in circulation.
Membrane lipids succumb easily to deleterious actions of reactive oxygen species [38]. The measurement of lipid peroxidation is a convenient method to monitor oxidative damage [39]. In the present study the increased levels of TBARS and hydroperoxides in plasma and liver of rats treated with CQ reflected the lipid peroxidation as the consequence of oxidative stress caused by CQ [7].
Treatment with THC protects the cells through attenuation of lipid peroxidation and decreased the production of free radical derivatives, as evident from the decreased levels of plasma and liver TBARS and hydroperoxides. Thus THC offered protection against oxidative stress [40] by scavenging of free radicals [41]. Curcumin also significantly reduced the lipid peroxidation by its ability to scavenge the free radicals [42].
Non-enzymic antioxidants such as reduced glutathione, vitamin C and vitamin E play an excellent role in protecting the cells from oxidative damage [43, 44]. It is well established that GSH in blood keeps up the cellular levels of the active forms of vitamin C and vitamin E by neutralizing the free radicals. When there is a reduction in the GSH the cellular levels of vitamin C is also lowered, indicating that GSH, vitamin C, and vitamin E are closely interlinked to each other [45]. In agreement with this report, the decreased levels of GSH, vitamin C and vitamin E on CQ administration were observed in our study. Administration of THC and curcumin to CQ treated rats, maintained the level of non-enzymic antioxidants to near normal, by the possible role of THC [11] and curcumin in improving the GSH status.
The enzymic antioxidant defense systems are the nature protector against lipid peroxidation. SOD, CAT and GPx enzymes are important scavengers of superoxide ion and hydrogen peroxide. These enzymes prevent generation of hydroxyl radical and protect the cellular constituents from oxidative damage [46]. The decreased activities of SOD, CAT and GPx were observed in the CQ administered rats suggesting the increased lipid peroxidation. The increased activity of SOD, CAT and GPx in THC and curcumin administered rats may result from the scavenging of the radicals generated by CQ induced lipid peroxidation, thereby decreasing the utilization of these antioxidant enzymes to reduce the CQ induced oxidative threat. This might be responsible for the increased activities of antioxidant enzyme on administration of THC and curcumin.
The histopathological observations in CQ - treated rats showed feathery degeneration and/microvesicular type of fatty generation with sinusoidal dilation and focal necrosis. This could be due to the formation of highly reactive radicals because of oxidative threat caused by CQ. The accumulated hydroperoxides can cause cytotoxicity, which is associated with the peroxidation of membrane phospholipids by lipid hydroperoxide [47, 48]. All these changes were very much reduced histopathologically in rats treated with CQ and THC as well as in curcumin treated rats. As the alterations produced in the GSH indicates involvement of deleterious oxidative changes, increased levels of GSH would therefore be important in protecting cells against toxicity. Based on the above results, it could be concluded that THC is a hepato-stimulant and exerts a significant hepatoprotection against chloroquine-induced toxicity.
In conclusion, the present investigation indicates that THC and curcumin exerts significant protection against CQ induced toxicity by its ability to ameliorate the lipid peroxidation through the free radicals scavenging activity, which enhanced the levels of antioxidant defense system. Our study also showed that THC has greater effect than curcumin, in CQ induced lipid peroxidation because THC is the rapidly metabolized product of curcumin during absorption from the intestine [9, 49]. Therefore THC is suggested to be helpful in attenuation of CQ induced lipid peroxidation and showed more prominent effect than curcumin.
References
Issacson D, Elgart M, Tunner ML: Antimalarial in dermatology. Int J Dermatol, 1982; 21: 379-395.
Augustijus P, Verbeke N: Stereoselective phoramacokinetic properties of chloroquine de-ethyl-chloroquine in humans. Clin Pharmacokinet, 1993; 24: 259-269.
Ekpechi OL, Okoro A: Chloroquine causes unpleasant pruritus in Nigerians. Arcb Derm, 1964; 89: 631.
Pari L, Murugavel P: Protective effect of a - lipoic acid against CQ induced hepatotoxicity in rats. J Appl Toxicol, 2004; 24: 21-26.
Dass EE, Shah KK: Paracetamol and conventional antimalarial drugs induced hepatotoxicity and its protection by methionine in rats. Ind J Exp Biol, 2000; 38: 1138-1142.
Koranda FC: Antimalarials, J Am Acad Dermatol, 1981; 4: 650-655.
Magwere T, Saik YS, Hasler JA: Effect of chloroquine treatment on antimalarial enzymes in rat liver and kidney. Free Rad Biol Med, 1997; 22: 321-327.
Murugavel P, Pari L: Attenuation of chloroquine-induced renal damage by a - lipoic acid: possible antioxidant mechanism. Ren Fail, 2004; 26: 515 - 522.
Osawa T, Sugiyama Y, Inayoshi M, Kawakishi S: Antioxidative activity of Tetrahydrocurcumin. Biosci Biotechnol Biochem, 1995; 59: 1609-1612.
Sugiyama Y, Kawakishi S, Osawa T: Involvement of the b-diketone moiety in the antioxidant mechanism of tetrahydrocurcuminoids. Biochem Pharmacol, 1996; 52: 519-525.
Majeed M, Badmaev V, Uma S, Rajeuderan JR: Curcuminoids Antioxidant Phytonutrients. Nutriscience Publishers. New Jersey, 1995; 1: 24-33.
Naito M, Normura H, Kodama M, Kato Y, Osawa T: The protective effect of tetrahydrocurcumin on oxidative stress in cholesterol-fed rabbits. J Atheroscler Thromb, 2002; 9: 243-250.
Nakamura, Y: Inhibitory effect and Tetrahydrocurcuminoids on the tumor promoter induced reactive oxygen species generation in leucocyte, in vitro and in vivo. Jap J Cancer Res, 1998; 89: 361-370.
Pari, Murugan P: Protective role of Tetrahydrocurcumin (THC) against erythromycin estolate induced hepatotoxicity. Pharmacol Res, 2004; 49: 481-486.
Rajakrishnan V, Jayadeep A, Arun OS: Changes in the prostaglandin levels in alcohol toxicity. Effect of curcumin and N-acetyl cysteine. J Nutr Biochem, 2000; 11: 509-514.
Reitman S, Frankel SA: Colorimetric method for the determination of serum oxaloacetic and glutamic pynivate transaminass. Am. J. Clin. Pathol. 1957; 28: 56-63.
King EJ, Armstrong AR: Determination of Serum and bile phosphatase activity J Canad. Med. Assoc, 1934; 31: 376-378.
Malloy E, Evelyn K: The determination of bilirubin with the photoelectric colorimeter. J. Biol. Chem. 1937, 119, 481-485.
Folch J, Less M, Solane SGH: A simple method for isolation and purification of total lipids from animal tissues. J Biol Chem, 1957; 226: 497-509
Zlatkis A, Zak B, Boyle GJ: A Simple method for determination of serum cholesterol. J Clin Med, 1953; 41: 486-492.
Foster LB, Dunn RT : Stable reagents for determination of serum triglycerides by colorimetric hantzsch condensation method. Clin Chem, 1973; 18: 338-340.
Falholt K, Falholt W, Lund B: An aasy colorimetric method for routine determination of free fatty acids in plasma. Clin Chem Acta, 1973; 46: 105-111.
Zilversmit BB, Davis AK: Micro determination of plasma phospholipids by TCA Precipitation. J Lab Clin Med, 1950; 35: 155-161.
Nichans WG, Samuelson D: Formation of malondialdehyde from phospholipids arrachidonate during microsomal lipid peroxidation. Eur J Biochem, 1968; 6:126-30.
Jiang ZY, Hunt JV Wolff SD: Ferrous sulphate oxidation in the presence of xylenol orange for detection of lipid hydroperoxide in low-density lipoprotein. Aual Biochem, 1992; 202: 384-389.
Ellman GC: Tissue sulfhydryl groups. Arch Biochem Biophys, 1959; 82: 70-77.
Omaye ST, Turbull TP, Sauberchich HC: Selected methods for determination of ascorbic acid in cells, tissues and fluids. Methods, Enzymol. 1979; 6: 3-11.
Desai ID: Vitamin E analysis method for animal tissues. Methods Enzymol, 1984; 105: 138-143.
Kakkar P, Das B, Viswanathan PN: A modified spectroscopic assay of superoxide dismutase . Indian J Biochem Biophys, 1984; 21:130-132.
Sinha KA: Colorimetric assay of catalase. Ann Biochem, 1972; 47: 389-394.
Rotruck JT, Pope AL, Ganther HF, swason AB: Selenium: Biochem role as a component of glutathione peroxidase. Science, 1973; 179: 588-590.
Lowry OH, Rose Brough MJ, Farr AL, Randall RJ: Protein measurement with folin-phenol reagent. J Biol chem, 1951; 193: 265-275.
Duncan BD: Multiple range tests for correlated and heteroscedastic means. Biometrics, 1957; 13: 359-364.
Sallie R, Tredger JM, Willam R: Drugs and the Liver, Biopharmaceutical Drug Dispos, 1991; 12: 251-259.
Farombi EO, Olowg BI Emerole GO: Effect of three structurally related antimalarial drugs on liver microsomal components and lipid peroxidation in rats. Comp Biochem Physiol, 2000; 126: 217-224.
Debra KF, Megan NL: Quinine induced hepatotoxicity. Ann Pharmacother, 1999; 33: 32-34.
Farombi EO, Emerole GO: Interference of common antimalarial drugs with some hepatic microsomal components and drug metabolism: potential implication for toxicity. South Afr J Sci, 1998; 94: 303-304.
Reiter RJ: Oxidative processes and antioxidative defuse mechanisms in the aging brain. FASEB, 1995; 9: 526-533.
Viani P, Cervato G, Fiorilli A, Cestaro B: Age related difference in synapatozomal peroxidative damage and membrane properties. J Neurochem, 1991; 56: 253-258.
Khopde SM, Priyadarisini KT,. Guha SN,. Satav JG, Venkatesan P Rao MV: inhibition of radiation-induced lipid peroxidation by tetrahydrocurcumin: possible mechanism by pulse radiolysis. Biosci Biotechnol Biochem, 2000; 64: 503-509.
Bonte F: Protective effect of curcuminoids on epidermal skin cells under free oxygen radical stress. Planta Med, 1997; 63: 265-266.
Venkatesan P, Rao MNA: J Pharm Pharmacol, 2000; 52: 1123-1128.
Pesh-Imam M, Reckuagel RO: Lipid peroxidation and the concept of anti-oxygenic potential; Vitamin E change in accute experimental CCl4, and ethanol induced liver injury. Toxicol Appl Pharamacol, 1977; 42: 463-475.
Aldrige WN: Mechanism of toxicity. New concepts are required in toxicology. Trends Pharmacol Sci, 1981; 2: 228-231.
Winkler BS: Unequivocal evidence is support of non-enzymatic redox coupling between glutathione/glutathione disulphide and ascorbic acid, dehydro ascorbic acid. Biochim Biophys Acta, 1992; 1117: 287-290.
Scott MD, Lubin BH, Zuo L, Kuypers FA: Erythrocyte defense against hydrogen peroxide : Preeminent importance of Catalase. J Lab Clin Med, 1991; 118: 7-16.
Kaneko T, Baba N, Matsuo M: Suppression of Lipid hydroperoixde – induced oxidative damage to cellular DNA by esculatin. Biol Pharm Bull, 2003; 26: 840-849.
Pacifici EH, McLeod LL, Peterson H, Savanian: Linoleic acid hydroperoxide induced peroxidation of endothelial cell phospholipids and cytotoxicity. Free Radic Biol Med, 1994; 17: 285-295.
Ravidranath V, Chandrasekara N: Absorption and tissue distribution of curcumin in rats. Toxicology, 1980; 16: 259-265.
Corresponding Author: Dr. L. Pari, Department of Biochemistry, Faculty of Science, Annamalai University, Annamalai Nagar-608 002, Tamil Nadu, India. paribala@sancharnet.in; paribalaji@rediffmail.com
Published by the Canadian Society for Pharmaceutical Sciences.
Copyright © 1998 by the Canadian Society for Pharmaceutical Sciences.
http://www.cspscanada.org