J Pharm Pharmaceut Sci (www.cspscanada.org) 8(1):39-46, 2005
Anti-diarrhoeal potential of asparagus racemosus wild root extracts in laboratory animals.
N. Venkatesan1, Vadivu Thiyagarajan1, Sathiya Narayanan1, Arokya Arul1, Sundararajan Raja2, Sengodan Gurusamy Vijaya Kumar3, Thandavarayan Rajarajan3, James Britto Perianayagam3*
1K.P. College of Pharmacy, Thiruvannamalai; 2Department of Pharmaceutical Technology, Faculty of Engineering and Technology, Jadavpur University, Kolkata; 3Faculty of Pharmaceutical Sciences, Guru Jambheshwar University, Hisar, IndiaReceived 4 October 2004, Revised 16 December 2004, Accepted 12 January 2005, Published 25 February 2005
PDF Version
Abstract
PURPOSE: Asparagus racemosus Wild root has been used traditionally in Ayurveda for the treatment of diarrhoea and dysentery. However, the claims of Ayurveda need to be validated by a suitable experimental model. Therefore, the present study was undertaken to evaluate the effect of ethanol and aqueous extracts of Asparagus racemosus for its antidiarrhoeal potential against several experimental models of diarrhoea in Albino Wistar rats. METHODS: The antidiarrhoeal activity of ethanol and aqueous extracts of Asparagus racemosus root was evaluated using castor oil-induced diarrhoea model in rats. Further, we evaluated the effect of ethanol and aqueous extracts on gastrointestinal tract motility after charcoal meal administration and PGE2 induced intestinal fluid accumulation (enteropooling). Loperamide was used as positive control. RESULTS: The plant extracts showed significant (P < 0.05) inhibitor activity against castor oil induced diarrhoea and PGE2 induced enteropooling in rats when tested at 200 mg/kg. Both extracts also showed significant (P < 0.001) reduction in gastrointestinal motility in charcoal meal test in rats. CONCLUSION: The results point out the possible anti-diarrhoeal effect of the plant extracts and substantiate the use of this herbal remedy as a non-specific treatment for diarrhoea in folk medicine.
Introduction
Diarrhoea has long been recognized as one of the most important health problems in the developing countries (1). Worldwide distribution of diarrhoea accounts for more than 5-8 million deaths each year in infants and small children less than 5 year. According to WHO estimation for the year 1998, there were about 7.1 million deaths due to diarrhoea (2). Secretory diarrhoea is the most dangerous symptom of gastrointestinal problems (3) and is associated with excessive defecation and stool outputs, the stools being of abnormally loose consistency (4).
Asparagus racemosus Wild (Liliaceae), commonly known as Satawari (Hindi) is a perennial shrub, with a tuberous root-stock, stems covered with recurved spines, linear leaves arranged in a tuft, white flowers and sweet-scented appears in October. The plant occurs through out India upto 1500 metres elevation. Asparagus racemosus is recommended in Ayurvedic texts for prevention and treatment of gastric ulcers as galatogogue and nervine tonic. The decoction of root has been used in blood diseases, diarrhoea, dysentery, cough, bronchitis and general debility (5-7).
Reports indicate that the pharmacological activities of root extracts include antiulcer (8), anti-tussive (9), antioxidant (10) and antibacterial activities (11). However, there is no scientific proof justifying the traditional use of Asparagus racemosus root in the treatment of diarrhoea. Hence, the present work was undertaken to evaluate its potential antidiarrhoeal efficacy in different experimental models of diarrhoea in albino rats.
Materials and Methods
Plant material
The roots of Asparagus racemosus Wild were collected from Uthangari, Tiruvannamalai district of Tamilnadu, India in March, 2002. The plant was identified by routine pharmacognostical studies including organoleptic tests, and macroscopic and microscopic observations. The voucher specimen (NV-168) has been retained in our laboratory for future reference. The collected roots were air-dried and pulverised using mechanical grinder.
Preparation and phytochemical study of extracts
The roots (500 g) were coarsely powdered and subjected to successive solvent extraction with 95% ethanol and water. A semi-solid extract was obtained after complete elimination of solvent under reduced pressure. The yield of both the extracts was 5.6±.45% and 6.2±0.32% respectively. The extracts were stored in desiccators and used for further experiment after suspending in aqueous Tween 80 solution (0.5%). The chemical constituents of the extracts were identified by qualitative chemical tests and further confirmed by thin layer chromatography study for the presence of alkaloids, sterols and/or terpenes and flavonoids.
Animals
Inbreed Albino Wistar rats of either sex weighing between 200 and 260 g were used. They were housed in polyacrylic cages and fed with standard rodent pellet diet and given water ad libutum. The animals were housed under standard laboratory environmental condition for an acclimatization period of 14 days prior to perform the experiments.
Castor oil induced diarrhoea
Rats were divided into eight groups (n = 6) and, fasted for 18 h and water was provided ad libitum. The ethanol and aqueous extracts of Asparagus racemosus (150, 200 and 250 mg/kg, p.o.) were administered orally to the first six groups of rats. One group received 10 ml/kg 0.5% v/v aqueous Tween 80 and served as a negative control. Another group received the standard drug loperamide (3 mg/kg, p.o.) as positive control. After 1 h of treatment, all the animals were challenged with 1 ml of castor oil orally, by gavage and observed for consistency of faecal material (12). The frequency of defecation was noted in transparent plastic dishes placed beneath the individual rat cages upto 4 h (13).
Gastrointestinal motility test
Rats were divided into four groups (n = 6) and fasted for 18 h before the experiment. Each animal was orally administered with 1 ml of charcoal meal (5% deactivated charcoal in 10% aqueous tragacanth) followed by oral administration of ethanol and aqueous extracts to the first two groups of animals in the dose of 200 mg/kg. The third group was treated with 0.5% v/v aqueous Tween 80 (10 ml/kg, p.o.) and served as a negative control. The fourth group received atropine (0.1 mg/kg, i.p.), as the positive control. Thirty minutes later, each animal was killed and the intestinal distance moved by the charcoal meal from the pylorus to caecum was measured and expressed as percentage of distance moved (14).
PGE2 -induced enteropooling
In this method rats were deprived of food and water for 18 h and placed in four cages, with six animals per cage. The first two groups were treated with 250 mg/kg dose of ethanol and aqueous extracts of Asparagus racemosus. The third group was treated with 1 ml of a 5% v/v ethanol in normal saline (i.p.) and then it was treated with 0.5% Tween 80 suspension, which served as a negative control. Immediately after the extract administration PGE2 (Astra Zeneca, India) was administered orally to each rat (100 mg/kg) in the first three groups. The fourth group was treated with PGE2 (100 mg/kg) as well as 0.5% Tween 80 suspension and served as the PGE2 control group. After 30 min following administration of PGE2, each rat was sacrificed and the whole length of the intestine from the pylorus to the caecum was dissected out, its content collected in a test tube, and the volume measured (14).
Statistical analysis
The data were analysed statistically using one-way analysis of variance followed by Dunnett's `t' test. The data are expressed as mean ± s.e.m. P-values less than 0.05 imply significance.
Results
Chemical analysis
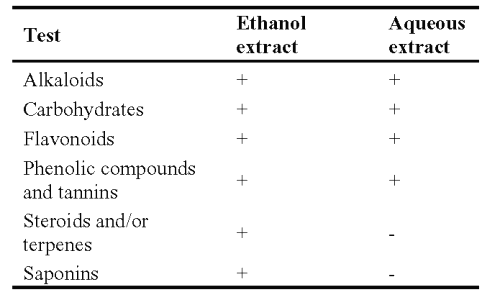
The results of the preliminary phytochemical screening of ethanol and aqueous extracts of A. racemosus root have been presented in Table 1.
Table 1: Phytochemical screening of A. racemosus, -, Absence; +, Presence.
Castor oil induced diarrhoea
Administration of castor oil produced characteristic semi-solid diarrhoea dropping in 18 h starved rats of the control group during the 4 h observation period (Table 2).
Table 2: Effect of ethanol and aqueous extracts of Asparagus racemosus on castor oil induced diarrhoea in rats. *The test drug, loperamide and vehicle were given p.o. Results are expressed as mean ± S.E.M., n = 6. Statistical significance test with control was done by Anova test. *P < 0.05, **P< 0.01 when compared to control
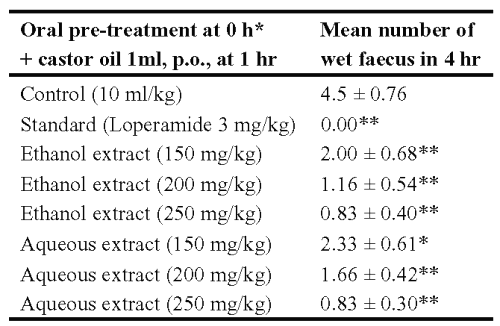
The ethanol and aqueous extracts at doses of 150, 200, 250 mg/kg showed significant (P < 0.001) reduction in the number of defecations over four hours when compared to that of untreated control rats; the activity was similar to that of loperamide (3 mg/kg), the standard anti-diarrhoeal agent. Both ethanol and aqueous extracts delayed the onset of diarrhoea and 100 and 80% of rats were protected against castor oil-induced diarrhoea at four hour, respectively.
Small intestinal transit
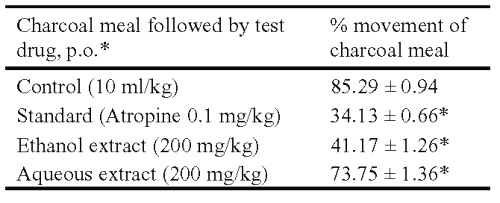
The ethanol and aqueous extracts decreased propulsion of the charcoal meal through the gastrointestinal tract at the oral dose of 200 mg/kg; as compared with control group (0.5% Tween 80). A similar reduction in the gastrointestinal transit of charcoal meal in rat was achieved with the intraperitoneal injection of atropine sulphate (0.1 mg/kg) (Table 3).
Table 3: Effect of ethanol and aqueous extracts of Asparagus racemosus on gastro intestinal transit in rats. *The test drug and vehicle were given p.o. and Atropine was given i.p. Results are expressed as mean ± S.E.M., n = 6. Statistical significance test with control was done by Anova test. *P< 0.001 when compared to control.
PGE2 -induced enteropooling
Both extracts significantly inhibited PGE2 induced enteropooling in rats at an oral dose of 250 mg/kg (Table 4).
Table 4: Anti-enteropooling effect of ethanol and aqueous extracts of Asparagus racemosus in rats. *The test drug and vehicle were given p.o. Results are expressed as mean ± S.E.M., n = 6. Statistical significance test with control was done by Anova test. aWith respect to ethanol in saline treatment. bWith respect to PGE2 treatment.
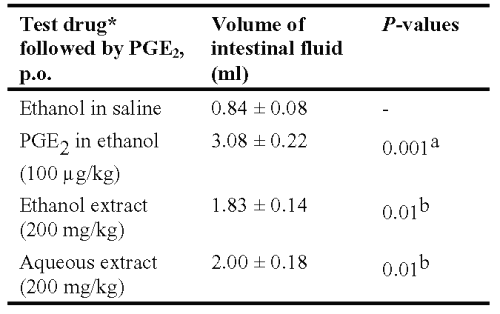
PGE2 induced a significant increase in the fluid volume of the rate intestine when compared with control animals received ethanol in normal saline.
Discussion
In developing countries, a quarter of infant and childhood mortality is related to the diarrhoea (15). The highest mortality rates have been reported to be in children less than five years of age. During the past decade oral dehydration therapy has reduced mortality from acute diarrhoeal disease, whereas chronic diarrhoea remains a life-threatening problem in those regions, in which malnutrition is a common co-existing and complication factor. Number of factors, such as infective, immunological and nutritional has been involved in the perpetuation of the diarrhoeal syndrome (16). Many plants conveniently available in India are used in traditional folklore medicine for the treatment of diarrhoea and dysentery. Of the indigenous plants used, Andrographis paniculata, Asparagus racemosus, Butea monosperma, Cassia auriculata, and others are mentioned (17). Several studies have shown that prior administration with some plant extracts had a protective effect on the intestinal tract (18-20). In the present study, ethanol and aqueous extracts of Asparagus racemosus that have not been studied so far, was evaluated for its anti-diarrhoeal potential against castor oil induced diarrhoea, gastrointestinal motility in charcoal meal test and PGE2 induced enteropooling in Albino Wistar rats.
The ethanol and aqueous extract of Asparagus racemosus exhibited significant anti-diarrhoeal activity against castor oil induced diarrhoea in rats. The extracts had a similar activity as loperamide, when tested at 200 and 250 mg/kg and statistically significant reduction in the frequency of defecation and the wetness of the faecal droppings when compared to untreated control rats.
It is widely known that castor oil or its active component ricinoleic acid induces permeability changes in mucosal fluid and electrolyte transport that results in a hypersecretory response and diarrhoea (21, 22). The experimental studies in rats demonstrated a significant increase in the portal venous PGE 2 concentration following oral administration of castor oil (23). Ricinoleic acid markedly increased the PGE 2 content in the gut lumen and also caused on increase of the net secretion of the water and electrolytes into the small intestine (24). The liberation of ricinoleic acid from castor oil results in irritation and inflammation of the intestinal mucosa, leading to release of prostaglandins, which stimulate motility and secretion (25). Inhibitors of prostaglandin biosynthesis delayed castor oil induced diarrhoea (12). In our unpublished preliminary study, both extracts exhibited significant anti-inflammatory activity in the carrageenan-induced rat paw oedema. Based on these observations, it seems reasonable to suggest that the anti-diarrhoeal effect of ethanol and aqueous extracts may be due to the inhibition of prostaglandin biosynthesis.
The extract appears to act on all parts of the intestine. Thus, it reduced, the intestinal propulsive movement in the charcoal meal treated model; at 200 mg/kg both extracts showed activity similar to that of atropine. Previous study shows that activated charcoal avidly absorbs drugs and chemicals on the surface of the charcoal particles thereby preventing absorption (26). Thus, gastrointestinal motility test with activates charcoal was carried out to find out the effect of ethanol and aqueous extracts on peristaltic movement. The results also show that the ethanol and aqueous extracts suppressed the propulsion of charcoal meal thereby increased the absorption water and electrolytes.
The extracts also significantly inhibited the PGE2 induced intestinal fluid accumulation (enteropooling). It has been shown that E type of prostaglandins cause diarrhoea in experimental animals as well as human beings (27). Their mechanism has been associated with dual effects on gastrointestinal motility as well as on water and electrolyte transport (28). PGE2 also inhibit the absorption of glucose, a major stimulus to intestinal absorption of water and electrolytes (29). These observations tend to suggest that both extracts at a dose of 250 mg/kg reduced diarrhoea by inhibiting PGE 2 induced intestinal accumulation of fluid.
Previous reports have demonstrated the antidiarrhoeal activity of tannin (30), flavonoids (31), alkaloids (32), saponins, reducing sugars and sterols and/or terpenes (33) containing plant extracts. The phytochemical analysis of the extracts showed the presence of alkaloids, saponins, flavonoids, sterols and /or terpenes and sugars. These constituents may responsible for the anti-diarrhoeal activity of A. racemosus extracts.
The antidiarrhoeal activity of flavonoids has been ascribed to their ability to inhibit intestinal motility and hydro-electrolytic secretion (34-36), which are known to be altered in this intestinal condition. In vitro and in vivo experiments have shown that flavonoids are able to inhibit the intestinal secretary response, induced by prostaglandins E2 (37). In addition, flavonoids present antioxidant properties (38) which are presumed to be responsible for the inhibitory effects exerted upon several enzymes including those involved in the arachidonic acid metabolism (39). The crude extract and a purified fraction of A. racemosus exhibited antioxidant activity against damage induced by gamma-radiation in rat liver (10). Sexana and Chourasia (40) reported isoflavone from the roots of A. racemosus. The preliminary phytochemical analysis of extracts also revealed the presence of flavonoids. As a consequence, it is possible to suggest that the antisecretary and antioxidant properties of flavonoid could contribute to the observed anti-diarrhoeal effect.
In some cases, it has been found that anti-diarrhoeal activity is associated with the antimicrobial (41). Earlier report indicates that the crude methanol extract of A. racemosus root exhibited significant anti-microbial activity against Escherichia coli, Salmonella typhimurium and Vibrio cholerae. (11). The three pathogens cause a variety of diseases including diarrhoea and gastroenteritis in human (42).
The results indicate that the ethanol and aqueous extract of Asparagus racemosus possesses significant anti-diarrhoeal activity due to its inhibitory effect both on gastrointestinal propulsion and fluid secretion. The data obtained are consistent with literature report on antidiarrhoeal activity of Asparagus pubescens root using gastrointestinal motility test and castor oil-induced diarrhoea and intraluminal accumulation of fluid in rats (43). The inhibitory effect of the extract justified the use of the plant as a non-specific anti-diarrhoeal agent in folk medicine. Further detailed investigations are underway to determine the exact phytoconstituents which are responsible for the antidiarrhoeal activity.
Acknowledgements
The authors are grateful to M/s Astra Zeneca Limited, Bangalore, India for providing free samples of PGE2. We are also thankful to Prof. P. Jayaraman for the taxonomic identification of the plant material.
References
Snyder, J.D. and Merson, M.H., The magnitude of the global problem of acute diarrhoea disease: A review of active surveillance of data. Bull WHO, 60: 605-613, 1982.
Park, K., Park’s Text book of Preventive and Social Medicine. Banarsidas Bharat Publishers, Jabalpur, pp. 122-175, 2000.
Fontaine, O., Diarrhoea and treatment. Lancet, 28: 1234-1235, 1988.
Aranda-Michel, J. and Gianella, R.A., Acute diarrhoea: A practical review. Am J Med, 106: 670-676, 1999.
Dey, A.C., Indian Medicinal Plants Used in Ayurvedic Preparations. Bishan Singh Mahendra Pal Singh, Dehradun, pp. 136-137, 1980.
Deokar, A.B., 125 Medicinal Plants Grown at Rajagaon. DS Manav Vikas Foundation, Pune, pp. 20-21, 1998.
Goyal, R.K., Singh, J. and Lal, H., Asparagus racemosus-An update. Indian J Med Sci, 57: 408-14, 2003.
Sairam, K., Priyambada, S., Aryya, N.C. and Goel, R.K., Gastroduodenal ulcer protective activity of Asparagus racemosus: an experimental, biochemical and histological study. J Ethanopharmacol, 86 : 1-10, 2003.
Mandal, S.C., Kumar, C.K.A., Mohana Lakshmi, S., Sinha, S., Murugesan, T., Saha, B.P. and Pal, M., Antitussive effect of Asparagus racemosus root against sufur dioxide-induced cough in mice. Fitoterapia. 71: 686-689, 2000.
Kamat, J.P., Boloor, K.K., Devasagayam, T.P. and Venkatachalam, S.R., Antioxidant properties of Asparagus racemosus against damage induced by gamma-radiation in rat liver mitochondria. J Ethanopharmacol, 71: 425-35, 2000.
Mandal, S.C., Nandy, A., Pal, M. and Saha, B.P., Evaluation of antibacterial activity of Asparagus racemosus Wild root. Phytother Res, 14: 118-119, 2000.
Awouters, F., Nimegeers, C.J.E., Lenaerts, F.M. and Janssen, P.A.J., Delay of castor oil diarrhoea in rats : a new way to evaluate inhibitors of prostaglandin biosynthesis. J Pharm Pharmacol. 30: 41-45, 1978.
Gnanasekar, N. and Perianayagam, J.B., Influence of sodium curcuminate on castor oil induced diarrhoea in rats. Indian J Pharmacol, 36(3); 177-178, 2004.
Gunakkunru, A., Padmanaban, K., Thirumal, P., Pritila, J., Parimala, G., Vengatesan, N., Gnanasekar, N., Perianayagam, J.B., Sharma, S.K. and Pillai, K.K., Anti-diarrhoeal activity of Butea monosperma in experimental animals. J Ethanopharmacol, 2004 (In Press).
Jousilahti, P., Madkour, S.M., Lambrechts, T. and Sherwin E., Diarrhoeal disease morbidity and home treatment practical in Egypt Public Health, 111 (1): 5-10, 1997.
Galvez, J., Sanchez de Medina, F., Jimenez, J., Torres, M.I., Fernandez, M.I., Nunez, M.C., Rios, A., Gil, A. and Zarzuelo, A., Effect of quercitrin on lactose-induced chronic diarrhoea in rats. Planta Med, 61: 302-306, 1995.
Chopra, R.N., Nayar, S.L. and Chopra, I.C., Glossary of Indian Medicinal Plants. Council of Scientific and Industrial Research, New Delhi, 1956.
Rani, S., Ahamed, N., Rajaram, S., Saluja, R., Thenmozhi, S. and Murugesan, T., Anti-diarrhoeal evaluation of Clerodendrum phlomidis Linn. leaf extract in rats. J Ethanopharmacol 68: 315‑319, 1999.
Majumdar, A.M., Upadhye, A.S. and Misar, A.V., Studies on anti-diarrhoeal activity of Jatropha curcus root extract in albino mice. J Ethanopharmacol, 70: 183-187, 2000.
Kumar, S., Dewan, S., Sangraula, H. and Kumar, V.L., Anti-diarrhoeal activity of the latex of Calotropis procera. J Ethanopharmacol, 76: 115-118, 2001.
Ammon, H.V., Thomas, P.J. and Phillips, S., Effect of oleic and recinoleic acid on net jejunal water and electrolyte movement. J Clin Inves, 53: 374-379, 1974.
Gaginella, T.S., Stewart, J.J., Olson, W.A. and Bass, P., Actions of ricinoleic acid and structurally related fatty acid on the gastro-intestinal tract II. Effects on water and electrolyte absorption in vitro. J Pharmacol Exp Ther, 195: 355-361, 1975.
Luderer, J.R., Dermers, I.M. and Hayes, A.T., Advances in Prostaglandin and Thromboxane Research. Raven Press, New York, pp. 1633-1638, 1980.
Beubler, E. and Juan, H., Effect of ricinoleic acid and other laxatives on net water flux and prostaglandin E release by the rat colon. J Pharm Pharmacol, 31: 681-685, 1979.
Pierce, N.F., Carpenter, C.C.J., Elliott, H.Z. and Greenough, W.B., Effects of prostaglandins, theophylline and cholera exotoxin upon transmucosal water and electrolyte movement in canine jejunum. Gastroenterology, 60: 22-32, 1971.
Levy, G., Gastrointestinal clearance of drugs with activated charcoal. New Eng J Med, 307: 676-678, 1982.
Eakins, K.E., Sanner, J.M., Prostaglandins Antagonists, in Karim SMM (ed), Prostaglandins Progress in Research. Wiley-Interscience, New York, pp. 263-264, 1972.
Dajani, E.Z., Roge, E.A.N., Bertermann, R.E., Effects of E prostaglandins, diphenoxylate and morphine on intestinal motility in vivo. Eur J Pharm, 34: 105-113, 1975.
Jaffe, B.M., Prostaglandins and serotonin: Nonpeptide diarrhoeogenic hormones. World J Surg, 3: 565-578, 1979.
Mukherjee, P.K., Saha, K., Murugesan, T., Mandal, S.C., Pal, M. and Saha, B.P., Screening of anti-diarrhoeal profile of some plant extracts of a specific region of Wet Bengal, India. J Ethanopharmacol, 60: 85-89, 1998.
Galvez, J., Zarzuelo, A., Crespo, M.E., Lorente, M.D., Ocete, M.A. and Jimenez, J., Antidiarrhoeic activity of Euphorbia hirta extract and isolation of an active flavonoid constituent. Planta Med, 59: 333-336, 1993.
Gricilda Shoba, F. and Molly Thomas, Study of anti-diarrhoeal activity of four medicinal plants in castor oil-induced diarrhoea. J Ethanopharmacol, 76: 73-76; 2001.
Otshudi, A.L., Vercruysse, A. and Foriers, A., Contribution to the ethanobotanical, phytochemical and pharmacological studies of traditionally used medicinal plants in the treatment of dysentery and diarrhoea in Lomela area (DRC). J Ethanopharmacol, 71: 411‑423, 2000.
Di Carlo, G., Autore, G., Izzo, A.A., Maibline, P., Mascolo, N., Viola, P., Diurno, M.V. and Capasso, F., Inhibition of intestinal motility and secretion by flavonoids in mice and rats: Structure activity relationships. J Pharm Pharmacol, 45: 1054‑1059, 1993.
Galvez, J., Crespo, M.E., Jimenez, J., Suarez, A. and Zarzuelo, A., Anti-diarrhoeic activity of quercitrin in mice and rats. J Pharm Pharmacol, 45: 157-159, 1993.
Rao, V.S.N., Santos, F.A., Sobreika, T.T., Souza, M.F., Melo, L.L. and Silveira, E.R., Investigations on the gastroprotective and antidiarrhoeal properties of ternatin, a tetramethoxyflavone from Egletes viscose. Planta Med, 63: 146-1497, 1997.
Sanchez de Medina, F., Galvez, J., Gonzalez, M., Zarzuelo, A. and Barrett, K.E., Effects of quercetin on epithelial chloride secretion. Life Sci, 61: 2049-2055, 1997.
Su, Y.L., Leung, L.K., Bi, Y.R., Huang, Y. and Chen, Z.Y., Antioxidant activity of flavonoids isolated from Scutellaria rehderiana. J Am Chem Soc, 77: 807-812, 2000.
Mora, A., Paya, M., Rios, J.L. and Alcaraz, M.J., Structure activity relationships of polymethoxy flavones and other flavonoids as inhibitors of non-enzymic lipid peroxiation. Biochem Pharmacol, 36: 317-322, 1990.
Saxena, V.K. and Chourasia, S., A new isoflavone from the roots of Asparagus racemosus. Fitoterapia, 72(3): 307-309, 2001.
Otshudi, A.L., Foriers, A., Vercruysse, A., Van Zeebroeck, A. and Lauwers, S., In vitro antimicrobial activity six medicinal plants traditionally used for treatment of dysentery and diarrhoea in Democratic Republic of Congo (DRC). Phytomedicine, 7: 167-172, 2000.
Jawet, Z.E., Melnick, J. L. and Adelberg, E.A., Review of Medical Microbiology. 14th ed., Lange Medical Publications Drawerl., Los Altos, CA, pp. 230-232, 1980.
Nawfor, P.A., Okwuasaba, F.K. and Binda, L.G., Antidiarrhoeal and antiulcerogenic effects of methanolic extract of Asparagus pubescens root in rats. J Ethnopharmacol, 72: 421-427, 2000.
Corresponding Author: James B. Perianayagam, Faculty of Pharmaceutical Sciences, Guru Jambheshwar University, Hisar-125,001, India. jamesjbp@rediffmail.com
Published by the Canadian Society for Pharmaceutical Sciences.
Copyright © 1998 by the Canadian Society for Pharmaceutical Sciences.
http://www.cspscanada.org