J Pharm Pharmaceut Sci (www.cspscanada.org) 8(1):94-101, 2005
Inhibitory effect of Gamibojungikgitang extract on mast cell-mediated allergic reaction in murine model.
Phil-Dong Moon, Ho-Jeong Na, Hyun-Ja Jeong, Seung-Heon Hong1
College of Pharmacy, Wonkwang University, Iksan, Jeonbuk, South Korea; Department of Pharmacology, College of Oriental Medicine, Kyung Hee University, 1 Hoegi-Dong, Dongdaemun-Gu, Seoul, South KoreaSu-Jin Kim
College of Oriental Medicine, Kyung Hee University, Hoegi-Dong, Dongdaemun-Gu, Seoul, South Korea; Division of Biological Sciences, College of Natural Science, Chonbuk National University, Jeonju, Jeonbuk, South KoreaHan-Jung Chae
Department of Pharmacology and CardioVascular Research Institute, Chonbuk National University School of Medicine, Jeonju, Jeonbuk, South KoreaHyung-Ryong Kim
Department of Dental Pharmacology and Nano Science & Technology Research Institute, School of Dentistry, Wonkwang University, Iksan, Jeonbuk, South KoreaJeong-On Choi, Si-Hyeong Lee, Jo-Young Shin
College of Oriental Medicine, Wonkwang University, Iksan, South KoreaHyung-Min Kim1
College of Oriental Medicine, Kyung Hee University, Hoegi-Dong, Dongdaemun-Gu, Seoul, South KoreaReceived 19 November 2004, Revised 18 January 2005, Accepted 3 February 2005, Published 15 April 2005
PDF Version
Abstract
PURPOSE: Gamibojungikgitang (GBIT) is an Oriental herbal prescription medication, which has been commonly used to treat allergic rhinitis in far Eastern countries including Korea, China, and Japan. Additionally, GBIT effectively treats ovarian cysts and improves ovarian functions by regulating both endocrine and metabolic activities. However, it has not been cleared how it prevents allergic diseases in experimental model. Here we report the effect of GBIT on mast cell-mediated allergic reactions. METHODS: The anti-anaphylactic effect of GBIT extract was studied against compound 48/80-induced systemic anaphylactic shock model in mice. Rat Peritoneal Mast Cells (RPMCs) were used to investigate the effect of GBIT extract on histamine release induced by compound 48/80. Passive cutaneous anaphylaxis activated by anti-dinitrophenyl IgE was used to know the effect of GBIT extract. In addition, human mast cell line HMC-1 cells culture supernatants that GBIT extract pretreated were assayed for IL-6 protein levels by enzyme-linked immunosorbent assay method. RESULTS: GBIT extract dose dependently inhibited compound 48/80-induced systemic anaphylactic shock. When GBIT extract was given as pretreatment at concentrations ranging 0.01-1 mg/ml, the histamine release from rat peritoneal mast cells induced by compound 48/80 was reduced in a dose-dependent manner. GBIT extract also inhibited passive cutaneous anaphylaxis activated by anti-dinitrophenyl IgE. In addition, GBIT extract inhibited phorbol 12-myristate 13-acetate + A23187-induced interleukin-6 secretion from human mast cell line HMC-1 cells. CONCLUSION: These results suggest a potential role for GBIT extract as a source of anti-anaphylactic agents for allergic disorders.
Introduction
Gamibojungikgitang (GBIT) is an Oriental herbal prescription medication, which has been commonly used to treat allergic rhinitis in far Eastern countries including Korea, China, and Japan. Additionally, GBIT effectively treats ovarian cysts and improves ovarian functions by regulating both endocrine and metabolic activities (1, 2). However, it has not been cleared how it prevents allergic diseases in experimental model.
In general, immediate-type hypersensitivity reactions that involve urticaria, allergic rhinitis and asthma are mediated by various chemical mediators released from mast cells (3). Histamine is one of the well characterized and potent vasoactive mediators implicated in the acute phase of immediate-type hypersensitivity reactions among the substances released on degranulation of mast cells (4, 5). Compound 48/80 is well-known histamine releaser (6).
The secretory responses of mast cells can be induced by aggregation of their cell surface-specific receptors for immunoglobulin E (IgE) by the corresponding antigen (7-9). It has been established that the anti-IgE antibody induces passive cutaneous anaphylaxis (PCA) as a typical in vivo model for immediate-type hypersensitivity reactions in anaphylactic reactions. Rat's skins are useful sites for studying PCA (10).
Activated mast cells can produce histamine as well as a wide variety of other inflammatory mediators and several proinflammatory and chemotactic cytokines such as tumor necrosis factor-alpha (TNF-a), interleukin (IL)-1, IL-4, IL-6, IL-8, and IL-13 (11-14). Modulation of these cytokines secretion from mast cells can provide us with a useful therapeutic strategy for allergic inflammatory disease. Recent studies have demonstrated that IL-6 up-regulates histamine production rather than increases its storage and is an important inducing factor for the expression of immunoglobulin E Fc epsilon RI (15). And Kikuchi et al . announced that IL-6 enhances IgE-dependent histamine release from human peripheral blood-derived cultured mast cells (16). Also, inflammatory cytokines (IL-6, IL-8, and TNF-a) production was induced via evoking of HIF-1 and NF-κB response under hypoxic condition on HMC-1 cells (17).
Present study has evaluated the effect of GBIT extract on the compound 48/80-induced systemic anaphylactic shock, histamine release from rat peritoneal mast cells (RPMCs), and anti-dinitrophenyl (DNP) IgE antibody-induced PCA. We also investigated the effect of GBIT extract on phorbol 12-myristate 13-acetate (PMA) + A23187-induced IL-6 secretion from human mast cell line HMC-1 cells.
mATERIALS AND mETHODS
Materials
Compound 48/80, anti-DNP IgE, DNP-human serum albumin (HSA), metrizamide, PMA, A23187, ο-phthaldialdehyde (OPA), and evans blue were purchased from Sigma Chemical Co. (St. Louis, MO, USA). The α-minimal essential medium was purchased from Flow Laboratories (Irvine, UK). Fetal bovine serum was purchased from Life Sciences (Grand Island, NY, USA). Recombinant IL-6, biotinylated IL-6, anti-human IL-6 was purchased from BD PharMingen (Torreyana Road, San Diego, CA, USA).
Animals
The original stock of male ICR mice (4 week old) and male Wistar rats (7 week old) were purchased from the Dae-Han Experimental Animal Center (Eumsung, Chungbuk, South Korea), and the animals were maintained at the College of Pharmacy, Wonkwang University. The rats were housed five to ten per cage in a laminar air-flow room maintained at a temperature of 22±1°C and relative humidity of 55±1% throughout the study. No animal was used more than once. The research was conducted in accordance with the internationally accepted principles for laboratory animal use and care as found in the US guidelines (NIH publication #85-23, revised in 1985).
Preparation of GBIT extract
GBIT extract which is a mixture of ten traditional drugs as shown in Table 1 was obtained from the Oriental drug store, Daehak Oriental Drugstore (Iksan, South Korea) and classified and identified by local experts. Amounts of the ten traditional drugs studied in this work were shown in Table 1. The ratios for combining were based on the previous report (2). Extract of GBIT was prepared by decocting the dried prescription of herbs with boiling distilled water (GBIT: water = 1 : 5). The extraction decocted for approximately 3 h has been filtered, lyophilized, and kept a 4°C. Dilutions were made in saline then filtered through 0.45-μm syringe filter.
Table 1: The ratio of the component in GBIT extract.

Basis for selection of dose
The Amounts of GBIT for adult Korean (average body weight 60 kg) are about 100-150 g. The yield of powdered extraction is commonly about 5% (w/w). Therefore, the basis for selection of dose is as follows.
100-150g × 5% = 5-7.5 g ® An adult Korean (average body weight 60 kg) takes 5-7.5 g at one time. ® 5-7.5 g / 60 kg = 0.08-0.13 g/kg ® about 0.1 g/kg. Thus, the dose of GBIT for an adult person can be 0.1 g/kg. The dose range of 0.01-1 g/kg was chosen to see the dose-dependency.
Compound 48/80-induced systemic anaphylactic reaction
Mice (n=3) were given an intraperitoneal injection of the mast cell degranulator compound 48/80 (8 mg/kg). GBIT extract was dissolved in saline and administered orally with sonde 1 h before the injection of compound 48/80. The period for observation of mortality was based on the control mice that had died in 18 min by compound 48/80. Mortality was monitored for 18 min after induction of anaphylactic reaction.
Preparation of RPMCs
RPMCs were isolated as previously described (18). In brief, rats were anesthetized by ether, and injected with 20 ml of Tyrode buffer B (NaCl, glucose, NaHCO 3 , KCL, NaH 2 PO 4 ) containing 0.1% gelatin (Sigma) into the peritoneal cavity; the abdomen was gently massaged for about 90 sec. The peritoneal cavity was carefully opened, and the fluid containing peritoneal cells was aspirated by Pasteur pipette. Then the peritoneal cells were sedimented at 150 x g for 10 min at room temperature and resuspended in Tyrode buffer B. Mast cells were separated from the major components of rat peritoneal cells (i.e. macrophages and small lymphocytes) according to the method described by Yurt et al . (19). In brief, peritoneal cells suspended in 1 ml of Tyrode buffer B were layered onto 2 ml of 2.25 x 10 2 mg/ml metrizamide (density 1.12 g/ml; Sigma) and centrifuged at room temperature for 15 min at 400×g. The cells remaining at the buffer-metrizamide interface were aspirated and discarded; the cells in the pellet were washed and resuspended in 1 ml of Tyrode buffer A (10 mM HEPES, 130 mM NaCl, 5 mM KCl, 1.4 mM CaCl 2 , 1 mM MgCl 2 , 5.6 mM glucose, 0.1% bovine serum albumin) containing calcium. Mast cell preparations were about 95% pure as assessed by toluidine blue staining. More than 97% of the cells were viable as judged by trypan blue uptake.
Histamine assay
Purified RPMCs were resuspended in Tyrode buffer containing calcium for the treatment with compound 48/80. RPMC suspensions (2 x 10 5 cells/ml) were preincubated for 10 min at 37°C before the addition of compound 48/80 for stabilization. The cells were preincubated with the GBIT extract (0.01-1 mg/ml) for 30 min, and then incubated for 20 min with compound 48/80 (6 μg/ml). The reaction was stopped by cooling the tubes in ice. The cells were separated from the released histamine by centrifugation at 400×g for 5 min at 4°C. Residual histamine in the cells was released by disrupting the cells with perchloric acid and centrifugation at 400×g for 5 min at 4°C. The histamine content was measured by the OPA spectrofluorometric procedure of Shore et al . (20). The fluorescent intensity was measured at 440 nm (excitation at 360 nm) in spectrofluorometer. The inhibition percentage of histamine release was calculated using the following equation:
% inhibition = (A - B) ×100 / A
where A is histamine release without GBIT extract and B is the histamine release with GBIT extract.
PCA reaction
IgE-dependent cutaneous reaction was generated by sensitizing the skin with an intradermal injection of anti-DNP IgE followed 48 h later with an injection of DNP-HSA into the mice tail vein. The DNP-HSA was diluted in phosphate-buffered saline (PBS). The mice were injected intradermally with 100 ng of anti-DNP IgE into each of three dorsal skin sites that had been shaved 48 h earlier. The sites were outlined with a water-insoluble red marker. Forty-eight hours later, each mouse received an injection of 200 ml of the 1:1 mixture of 1 mg/ml DNP-HSA in PBS and 4% Evans blue via the tail vein. One hour before this injection, GBIT extract was administered orally.
The mice were sacrificed 40 min after the intravenous challenge. The dorsal skin of the mouse was removed for measurement of the pigment area. The amount of dye was then determined colorimetrically after extraction with 0.5 ml of 1.0 mol/l KOH and 4.5 ml of a mixture of acetone and phosphoric acid (with the ratio of 5:13), based on the method of Katayama et al . (21). The absorbent intensity of the extraction was measured at 620 nm in a spectrofluorometer, and the amount of dye was calculated with the Evans blue measuring-line.
IL-6 assay
Human mast cell line (HMC-1) was grown in IMDM (Gibco BRL, USA) with 10% FBS (JRH Bioscience, USA) at 37°C in 5% CO 2 and 95% humidity. HMC-1 cells were pretreated with various concentration of GBIT extract (0.01-1 mg/ml) for 30 min prior to PMA + A23187 stimulation. Culture supernatants were assayed for IL-6 protein levels by enzyme-linked immunosorbent assay (ELISA) method. Cytokines were measured by a modified ELISA method as described (22). Sandwich ELISA for IL-6 was carried out in duplicate in 96-well ELISA plates (Nunc, Denmark) coated with each of 100 μl aliquots of mouse anti-human IL-6 monoclonal antibodies (BD PharMingen, Torreyana Road, San Diego, California, USA) at 1.0 μg/ml in PBS at pH 7.4 and was incubated overnight at 4°C. The plates were washed with PBS containing 0.05% Tween-20 (Sigma, St. Louis, MO, USA) and blocked with PBS containing 1% BSA, 5% sucrose and 0.05% NaN 3 for 1 h.
After additional washes, sample or recombinant IL-6 standards were added and incubated at 37°C for 2 h. After 2 h of incubation at 37°C, the wells were washed and then each of 0.2 μg/ml of biotinylated anti-human IL-6 were added and incubated at 37°C for 2 h. After washing the wells, avidin-peroxidase was added and plates were incubated for 30 min at 37°C. Wells were again washed and ABTS substrate (Sigma) was added. Color development was measured at 405 nm using an automated microplate ELISA reader. A standard curve was run on each assay plate using recombinant human IL-6 (BD PharMingen) in serial dilutions.
Statistical analysis
The results were expressed as mean ± S.E.M. for the number of experiments. Statistical analysis was performed by the Student's t -test to express the difference from control group.
Results
Effect of GBIT extract on compound 48/80-induced systemic anaphylaxis
To assess the contribution of GBIT extract in anaphylactic reactions, we first used the in vivo model of systemic anaphylaxis.
As shown in Table 2, an oral administration of saline as a control induced a fatal reaction in 100% of each group. When the GBIT extract was orally administered at concentrations of 0.01-1 g/kg 1 h before compound 48/80 injection, the mortality was dose-dependently reduced (Table 2).
Table 2: Effect of GBIT extract on compound 48/80-induced systemic anaphylactic reaction in mice.
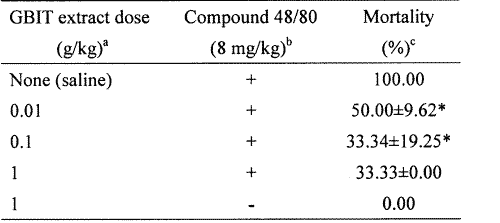
aThe groups of mice (n=3/group) were orally pretreated with 200 ml of saline or GBIT extract was given at various doses 1 h before the compound 48/80 injection.
bThe compound 48/80 solution was intraperitoneally given to the groups of mice.
cMortality (%) is presented as the `number of dead mice × 100 / Total number of experimental mice'. Each datum represents the mean±S.E.M. of four independent experiments. *P<0.05; significantly different from the control value.
Effect of GBIT extract on histamine release from RPMCs
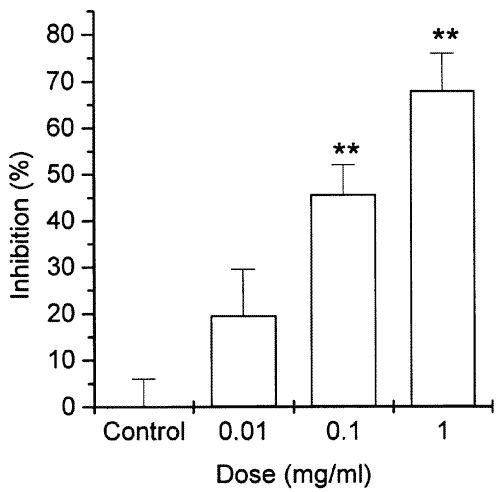
The inhibitory effect of GBIT extract on compound 48/80-induced histamine release from RPMCs is shown in Figure 1. GBIT extract dose-dependently inhibited compound 48/80-induced histamine release at concentrations of 0.01-1 mg/ml. At the concentration of 1 mg/ml, the inhibition rate reached up to 67.97%. In particular, GBIT extract significantly inhibited the compound 48/80-induced histamine release at concentrations of 0.1 and 1 mg/ml. The histamine intensity at the doses of 0.01-1 mg/ml was 11.16±1.36, 8.26±0.63, and 6.04±0.89, respectively. Control and spontaneous values were 13.34±1.16 and 2.31±0.51.
Figure 1: Effect of GBIT extract on compound 48/80-induced histamine release from RPMCs. RPMCs (2x10 5 cells) were preincubated with various concentrations of GBIT extract at 37°C for 10 min prior to incubation with compound 48/80. Each datum represents the mean+S.E.M. of three independent experiments. **P<0.01; significantly different from the control value.
Effect of GBIT extract on PCA
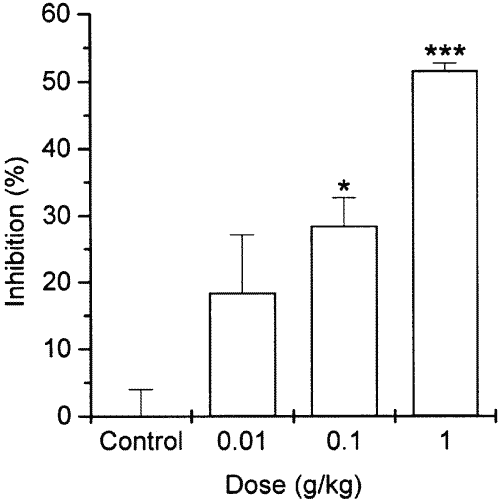
PCA is one of the most important in vivo models of anaphylaxis in local allergic reactions (23). When GBIT extract was orally administered to the mouse, the PCA reaction was inhibited in a dose-dependent manner (Figure 2). The best result was obtained at the dose of 1 g/kg. Amount of dye at the dose of 0.01-1 g/kg was 1.14±0.11, 1.01±0.05, and 0.68±0.02, respectively. The amount of dye in control mice was 1.14±0.02.
Figure 2: Effect of GBIT extract on 48 h PCA in mice. GBIT extract was administered orally 1 h prior to the challenge with antigen (DNP-HSA). Each datum represents the mean+S.E.M. of three independent experiments. *P<0.05, ***P<0.001; significantly different from the control value.
Effect of GBIT extract on IL-6 secretion from HMC-1 cells
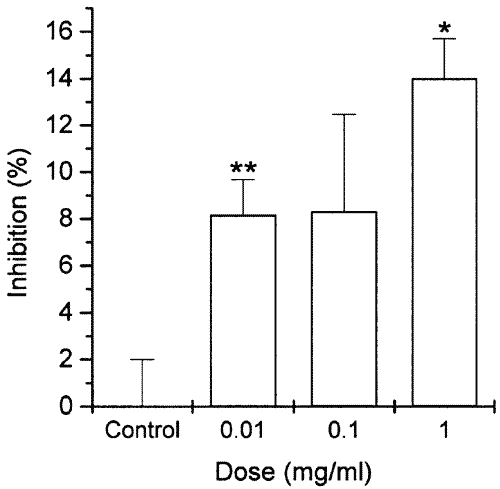
Our results showed that pretreatment of the cells with GBIT extract resulted in inhibition of IL-6 secretion. Inhibitory effect of GBIT extract was significant at the dose of 0.01 and 1 mg/ml (Figure 3). IL-6 production value at the doses of 0.01-1 mg/ml was 15.11±0.24, 15.08±0.66 and 14.17±0.27, respectively. Control and spontaneous values were 16.41±0.40 and 0.46±0.20.
Figure 3: Effect of GBIT extract on PMA + A23187-stimulated IL-6 secretion from HMC-1 cells. IL-6 levels in supernatant were measured using ELISA method. Each datum represents the mean+S.E.M. of three independent experiments. *P<0.05, **P<0.01; significantly different from the control value.
Discussion and Conclusion
Stimulation of mast cells with compound 48/80 is believed to initiate the activation of a signal transduction pathway, which leads to histamine release. There have been some reports that compound 48/80 is able to activate G proteins (24-26). Chadi et al. announced that compound 48/80 activates mast cell phospholipase D (PLD) via heterotrimeric GTP-binding proteins (27). They identified recombinant Gβ2γ2 subunit markedly synergized PLD activation by compound 48/80 in permeabilized RBL-2H3 cells. Murine mast cells are a good experimental model for the study on compound 48/80-induced histamine release (28). The report that compound 48/80 increased the permeability of the lipid bilayer membrane by causing a perturbation of the membrane (29) indicates that the membrane permeability increase may be an essential trigger for the release of mediators from mast cells. Our results showed that GBIT extract pretreatment profoundly affected compound 48/80-induced systemic anaphylaxis and histamine release (Table 2, Figure 1). Thus, it is possible to hypothesize that GBIT extract might act on the lipid bilayer membrane affecting the prevention of the perturbation induced by compound 48/80 and regulate the degranulation of the mast cells in mouse skin by stabilizing membrane fluidity.
GBIT extract-administered mouse was protected from IgE-mediated local allergic reaction. The mechanism of the protection against anti-DNP IgE may be suggested only in some particular conditions. It is conceivable that GBIT extract inhibits the initial phase of immediate type allergic reactions, probably through interference with the degranulation system.
HMC-1 cell line was a useful cell for studying cytokine activation pathways (30). Our data demonstrated that GBIT extract inhibited PMA + A23187-induced IL-6 secretion from HMC-1 cells. The effects of GBIT extract on mast cell cytokine secretion in vivo and the relative importance of mast cell as a source of chemotactic factor IL-6 during inflammatory and immune response are important areas for future studies. The amounts of GBIT extract that have been used (in vivo or in vitro) in this study are large, raising the possibility that the active agents in the extract represent a small component of the total mass.
GBIT is a complex prescription that is known to enhance spleen and stomach-qi on the basis of Oriental medicine theory (1). As a result of reinforcement of spleen and stomach-qi, GBIT can also reinforce wei-qi which guards the surface of the body against exopathogen (31,32). The main components of GBIT are known in the previous reports. Ginseng radix includes ginsenoside-Rb1 (G-Rb1), G-Rg1, and G-Ro (33), Astragali radix includes calycosin (34), Angelicae gigantis radix includes decursin (35), Pinelliae rhizoma includes beta-sitosterol (36), Atractylodis rhizoma alba includes atractylenolide I (37), Glycyrrhizae radix includes glycyrrhizin (38), Bupleuri radix includes saikosaponin a (39), Cimicifugae rhizoma includes acetylshengmanol xyloside and cimicifugoside H-1 (40), Aurantii nobilis pericarpium includes obacunone glucoside and nomilin glucoside (41), and Poria includes pachyman (42), respectively. It was, however, difficult to identify and separate specific compound through HPLC system from GBIT extract that consists of above ten traditional drugs.
In conclusion, the results obtained in the present study provide evidence that GBIT extract inhibited the mast cell-mediated anaphylactic reactions and inflammatory cytokines secretion. We believe that administration of GBIT extract may have a clinical applicability to the allergic disorders. However, further studies about active compounds of GBIT are needed.
Acknowledgements
This work was supported by a grant (#PF 2-13) from Plant Diversity Research Center of 21st Century Frontier Research Program funded by Ministry of Science.
References
Baek, S.H., A case report of ovarian cysts treatment with Gamibojungikgitang. J East-west Medicines, 24:1-16, 1999.
Kim, H.W., Lee, K.S., Song, B.K., Study on the effects of Gamibojungikgi Tang on ovarian reaction and pregnancy in obesity mice. J Oriental Gynecology
Miescher, S.M., Vogel, M., Molecular aspects of allergy. Mol Aspects Med, 23:413-462, 2002.
Petersen, L.J., Mosbech, H., Skov, P.S., Allergen-induced histamine release in intact human skin in vivo assessed by skin microdialysis technique: characterization of factors influencing histamine releasability. J Allergy Clin Immun, 97:672-679, 1996.
Moon, P.D., Na, H.J., Kim, H.M., Action of enzyme food, Green Life Enzyme of systemic and local anaphylaxis. Orient Pharm Exp Med, 3:46-50, 2003.
Kim, M.S., Na, H.J., Han, S.W., Jin, J.S., Song, U.Y., Lee, E.J., Song, B.K,, Hong, S.H., Kim, H.M., Forsythia fructus inhibits the mast-cell-mediated allergic inflammatory reactions. Inflammation, 27:129-135, 2003.
Alber, G., Miller, L., Jelsema, C., Varin-Blank, N., Metzger, H.. Structure-function relationships in the mast cell high affinity receptor for IgE. Role of the cytoplasmic domains and of the beta subunit. J Biol Chem, 266:22613-22620, 1991.
Hong, S.H., Jeong, H.J., Kim, H.M., Inhibitory effects of Xanthii fructus extract on mast cell-mediated allergic reaction in murine model. J Ethnopharmacol, 88:229-234, 2003.
Metzger, H., Alcaraz, G., Gogman, R., Kinet, J.P., Pribluda, V., Quarto, R., The receptor with high affinity for immunoglobulin E. Annu Rev Immunol, 4:419-470, 1986.
Na, H.J., Jeong, H.J., Bae, H., Kim, Y.B., Park, S.T., Yun, Y.G., Kim, H.M., Tongkyutang inhibits mast cell-dependent allergic reactions and inflammatory cytokine secretion. Clin Chim Acta, 319:35-41, 2002.
Artuc, M., Hermes, B., Steckelings, U.M., Grutzkau, A., Henz, B.M., Mast cells and their mediators in cutaneous wound healing--active participants or innocent bystanders? Exp Dermatol. 8:1-16, 1999.
Royer, B., Varadaradjalou, S., Saas, P., Gabiot, A.C., Kantelip, B., Feger, F., Guillosson, J.J., Kantelip, J.P., Arock, M., Autocrine regulation of cord blood-derived human mast cell activation by IL-10. J Allergy Clin Immun, 108:80-86, 2001.
Royer, B., Varadaradjalou, S., Saas, P., Guillosson, J.J., Kantelip, J.P., Arock, M., Inhibition of IgE-induced activation of human mast cells by IL-10. Clin Exp Allergy, 31:694-704, 2001.
Stassen, M., Muller, C., Arnold, M., Hultner, L., Klein-Hessling, S., Neudorfl, C., Reineke, T., Serfling, E., Schmitt, E., IL-9 and IL-13 production by activated mast cells is strongly enhanced in the presence of lipopolysaccharide: NF-kappa B is decisively involved in the expression of IL-9. J Immunol, 166:4391-4398, 2001.
Conti, P., Kempuraj, D., Di Gioacchino, M., Boucher, W., Letourneau, R., Kandere, K., Barbacane, R.C., Reale, M., Felaco, M., Frydas, S., Theoharides, T.C., Interleukin-6 and mast cells. Allergy Asthma Proc, 23:331-335, 2002.
Kikuchi, T., Ishida, S., Kinoshita, T., Sakuma, S., Sugawara, N., Yamashita, T., Koike, K., IL-6 enhances IgE-dependent histamine release from human peripheral blood-derived cultured mast cells. Cytokine, 20:200-209, 2002.
Jeong, H.J., Chung, H.S., Lee, B.R., Kim, S.J., Yoo, S.J., Hong, S.H., Kim, H.M., Expression of proinflammatory cytokines via HIF-1alpha and NF-kappaB activation on desferrioxamine-stimulated HMC-1 cells. Biochem Bioph Res Co, 306:805-811, 2003.
Jippo-Kanemoto, T., Kasugai, T., Yamatodani, A., Ushio, H., Mochizuki, T., Tohya, K., Kimura, M., Nishimura, M., Kitamura, Y., Supernormal histamine release and normal cytotoxic activity of beige (Chediak-Higashi syndrome) rat mast cells with giant granules. Int Arch Allergy Imm, 100:99-106, 1993.
Yurt, R.W., Leid, R.W., Austen, K.F., Native heparin from rat peritoneal mast cells. J Biol Chem, 252:518-521, 1977.
Shore, P.A., Burkhalter, A., Cohn, V.H., A method for fluorometric assay of histamine in tissues. J Pharmacol Exp Ther, 127:182-186, 1959.
Katayama, S., Shionoya, H., Ohtake, S., A new method for extraction of extravasated dye in the skin and the influence of fasting stress on passive cutaneous allergy in guinea pigs and rats. Microbiol Immunol, 22:89-101, 1978.
Kim, H.M., Lee, Y.M., Role of TGF-beta1 on the IgE-dependent anaphylaxis reaction. J Immunol, 162:4960-4965, 1999.
Wershil, B.K., Merkori, Y.A., Murakami, T., Galli, S.J., 125I-fibrin deposition in IgE dependent immediate-type hypersensitivity reactions reaction in mouse skin: demonstration of the role of mast cells using genetically mast cell-deficient mice locally reconstituted with cultured mast cells. J Immunol, 139:2605-2614, 1987.
Mousli, M.C., Bronner, C., Landry, Y., Bockaert, J., Rouot, B., Direct activation of GTP-binding regulatory proteins (G proteins) by substance P and compound 48/80. FEBS Lett, 25:260-262, 1990.
Mousli, M.C., Bronner, C., Bockaert, J., Rouot, B., Landry, Y., Interaction of substance P, compound 48/80 and mastoparan with a-subunit C-terminal of G protein. Immunol Lett, 25:355-358, 1990.
Shin, T.Y., Lee, J.K., Effect of Phlomis umbrosa root on mast cell-dependent immediate-type allergic reactions by anal therapy. Immunopharm Immunot, 25:73-85, 2003.
Chadi, A., Fraundorfer, P.F., Beaven, M.A., Compound 48/80 activates mast cell phospholipase D via heterotrimeric GTP-binding proteins. J Pharmacol Exp Ther, 292:122-130, 2000.
Alfonso, A., Cabado, A.G., Vieytes, M.R., Botana, L.M., Functional compartments in rat mast cells for cAMP and calcium on histamine release. Cell Signal, 12:343-350, 2000.
Tasaka, K., Mio, M., Okamoto, M., Intracellular calcium release induced by histamine releasers and its inhibition by some antiallergic drugs. Ann Allerg Asthma Im, 56:464-469, 1986.
Sillaber, C., Bevec, D., Buttrerfield, J.H., Heppner, C., Valenta, R., Scheiner, O., Kraft, D., Lechner, K., Bettelheim, P., Valent, P., Tumor necrosis factor alpha and interleukin-1 beta mRNA expression in HMC-1 cells: differential regulation of gene product expression by recombinant interleukin-4. Exp Hematol, 21:1271-1275, 1993.
Yuan, Y.X. et al., Chinese-English Dictionary of Traditional Chinese Medicine. The people’s Medical Publishing House, Beijing, pp 72-73, 1997.
Heo, J., Donguibogam. Bubin Publishers, Seoul, pp 157-158, 1999.
Matsuda, H., Yamazaki, M., Asanuma, Y., Kubo, M., Promotion of hair growth by ginseng radix on cultured mouse vibrissal hair follicles. Phytother Res, 17:797-800, 2003.
Nakamura, T., Hashimoto, A., Nishi, H., Kokusenya, Y., Investigation on the marker substances of crude drugs in formulations. I. Marker substances for the identification of astragali radix in kampo and drinkable preparations. Yakugaku Zasshi, 119:391-400, 1999.
Kang, S.Y., Lee, K.Y., Park, M.J., Kim, Y.C., Markelonis, G.J., Oh, T.H., Kim, Y.C., Decursin from Angelica gigas mitigates amnesia induced by scopolamine in mice. Neurobiol Learn Mem, 79:11-18, 2003.
Wu, H., Su, J., Cai, B., Zhang, L., Ye, D., Effect of ginger-processing on beta-sitosterol and total alkaloid contents in Rhizoma Pinelliae. Zhongguo Zhong Yao Za Zhi, 20:662-664, 702-703, 1995.
Zhang, Y., Xu, S., Lin, Y., Gastrointestinal inhibitory effects of sesquiterpene lactones from Atractylodes macrocephala. Zhong Yao Cai, 22:636-640, 1999.
Kim, S.C., Byun, S.H., Yang, C.H., Kim, C.Y., Kim, J.W., Kim, S.G., Cytoprotective effects of Glycyrrhizae radix extract and its active component liquiritigenin against cadmium-induced toxicity (effects on bad translocation and cytochrome c-mediated PARP cleavage). Toxicology, 197:239-251, 2004.
Jung, D.W., Sung, C.K., Production of antibody against saikosaponin a, an active component of bupleuri radix. Arch Pharm Res, 21:135-139, 1998.
Sakurai, N., Nagai, M., Chemical constituents of original plants of Cimicifugae rhizoma in Chinese medicine. Yakugaku Zasshi, 116:850-865, 1996.
Tian, Q., Ding, X., Screening for limonoid glucosides in Citrus tangerina (Tanaka) Tseng by high-performance liquid chromatography-electrospray ionization mass spectrometry. J Chromatogr A, 874:13-9, 2000.
Hattori, T., Hayashi, K., Nagao, T., Furuta, K., Ito, M., Suzuki, Y., Studies on antinephritic effects of plant components (3): Effect of pachyman, a main component of Poria cocos Wolf on original-type anti-GBM nephritis in rats and its mechanisms. Jpn J Pharmacol, 59:89-96, 1992.
Corresponding Authors: Hyung-Min Kim and Seung-Heon Hong, Department of Pharmacology, College of Oriental Medicine, Kyung Hee University, 1 Hoegi-Dong, Dongdaemun-Gu, Seoul, 130-701, South Korea. hmkim@khu.ac.kr; Department of Oriental Pharmacy, College of Pharmacy, Wonkwang University, Iksan, Jeonbuk, 570-749, South Korea. jooklim@wonkwang.ac.kr
Published by the Canadian Society for Pharmaceutical Sciences.
Copyright © 1998 by the Canadian Society for Pharmaceutical Sciences.
http://www.cspscanada.org