J Pharm Pharmaceut Sci (www.cspscanada.org) 8(1):102-106, 2005
An anti-inflammatory and anti-nociceptive effects of hydroalcoholic extract of Satureja khuzistanica Jamzad extract.
Massoud Amanlou, Fariba Dadkhah, Alinazar Salehnia, Hassan Farsam1
Department of Medicinal Chemistry, Faculty of Pharmacy, Tehran University of Medical Sciences, Tehran, IranAhmad Reza Dehpour
Department of Pharmacology, Faculty of Medicine, Tehran University of Medical Sciences, Tehran, IranReceived 2 November 2004, Revised 17 January 2005, Accepted 4 February 2005, Published 20 April 2005
PDF Version
Abstract
PURPOSE: Satureja khuzistanica Jamzad (Lamiaceae) is an endemic plant that widely distributed in the southern parts of Iran. This plant has been used as analgesic and antiseptic among the inhabitants of southern parts of Iran. METHODS. The Satureja khuzistanica hydroalcoholic extract was prepared and its anti-inflammatory and anti-nociceptive effects were investigated using the carrageenan-induced rat paw edema and formalin test. RESULTS. A similar anti-inflammatory activity was seen between S. khuzistanica hydroalcoholic extract (150 mg/kg; i.p.) and indomethacin (4 mg/kg; i.p.) in carrageenan test. The extract showed anti-nociceptive activity in a dose-dependent (10-150 mg/kg; i.p.) manner at the second phase of formalin test which was comparable with morphine (3 mg/kg; i.p.). CONCLUSION. This study confirms that anti-inflammatory and anti-nociceptive properties of S. khuzistanica are comparable to those of indomethacin and morphine. Presence of flavonoids, steroids, essential oil, mainly carvacrol and tannin might be responsible for anti-inflammatory and anti-nociceptive activities of this plant.
Introduction
Inflammation is a pathophysiological response of living tissues and a defense mechanism (1). Numerous studies have been carried out to develop more powerful anti-inflammatory drugs with lesser side effects. Satureja khuzistanica Jamzad (marzeh khuzistani in Persian, family of Lamiaceae) is an endemic plant that widely distributed in the southern parts of Iran (2). It is a subshrub, branched stem ± 30 cm high, densely leafy, broadly ovaiate-orbicular covered with white hairs. Base of the leaves is attenuate and petioliform. Each verticillaster has 2-8 flowers, shortly pedunculate and remote.
Botanically this species is close to S. edmondi Briquet but differs from it in having erect and branched stems, verticillasters are shortly pedunculate (2, picture 1).

Picture 1. Satureja khuzistanica Jamzad
This plant has been used as analgesic and antiseptic among the inhabitants of southern parts of Iran. Composition of the essential oils of wild and cultivated S. khuzistanica and the antioxidant, antidiabetic, anti-hyperlipidemic and reproduction stimulatory properties of this plant have been recently reported from Iran (3-5).
No work has been carried out on the anti-inflammatory and anti-nociceptive effects of this species. Keeping this in view, the present study has been undertaken to evaluate the anti-inflammatory and anti-nociceptive effects of the hydroalcoholic extract of this species using the carrageenan-induced rat paw edema and formalin test respectively.
mATERIALS AND mETHODS
Plant material and extraction procedure
S. khuzistanica aerial parts were collected from Khoramabad, Lorestan Province, Iran in August 2002 at an altitude of 1170 m. The plant was authenticated by Herbal Museum of the Faculty of Pharmacy, Tehran University of Medical Sciences, Tehran, Iran. A voucher specimen (No. 58416) has been deposited at the Herbarium of the Research Institute of Forests and Ranglands (TARI), Tehran, Iran.
The aerial parts of S. khuzistanica were chopped and air-dried under shade and then powdered and stored in an airtight container. S. khuzistanica hydroalcoholic extract (SKHE) were prepared with aqueous ethanol (50%; v/v) by percolation at room temperature for 24 h and concentrate at low pressure to constant weight (yield: 14.5 %).
Animals
Male albino Wistar rats (190-210 g) were obtained from the central animal house of the Razi Institute in Iran. They were kept at standard environmental conditions (12/12-h light/dark cycle) at the animal house of the Department of Pharmacology in the Faculty of Medicine, Tehran University of Medical Sciences (TUMS) and were allowed free access to food and water. Before each test, the animals were fasted for 24 h with free access to water. The rats were randomly divided into test and control groups, each group consisted of age and weight matched rats (n =9). Each animal tested once. The experimental protocol was approved by the animal care review committee of TUMS.
Formalin test
Male Albino Wistar rats, 190-210 g, were kept in Plexiglas cages with free access to food and water. Testing took place in the middle of the light period of a 12:12-h light:dark cycle. The SKHE and/or morphine sulfate were suspended in vehicle (1% carboxymethylcellulose (CMC) in saline) and administered interaperitoneally (i.p.) at a dose of 10, 100, 150 mg/kg for SKHE and 3 mg/kg for morphine in a volume of 1.5-2 ml for each rat (6). Control group received vehicle (2 ml; i.p.).
The anti-nociceptive activity of the tested compounds was determined using the formalin test as described by Dubuission & Dennis (7). One hour before testing, the animal was placed in a standard cage (30x12x13 cm) that served as an observation chamber. A total of 20 l of 1% formalin was injected into the plantar surface of the left hindpaw. The rat was observed for 60 min after the injection of formalin, and the amount of time spent licking the injected hindpaw was recorded. The first 5 min post formalin injection was considered as the early phase and the period between 15-60 min as late phase. The test compounds were administered (i.p.) 30 min before injection of formalin.
Carrageenan test
The anti-inflammatory activity of SKHE was determined by the carrageenan-induced edema test in the hindpaws of rats using the technique described by Niemegeer et al. (8). Male Wistar rats weighing 190-210 g were fasted for 24 h before the experiment with free access to water. Fifty ml of a 1% suspension of carrageenan (Sigma-Aldrich, St. Louis, MO, USA) in saline prepared 1 h before each experiment was injected into the plantar side of hindpaw of the rats.
The SKHE and indomethacin were suspended in vehicle (1% CMC) and administered i.p. at a dose of 100 and 150 and 4 mg/kg respectively in a volume of 1.5-2 ml for each rat (6). Control group received vehicle (1 % CMC, 2 ml; i.p.). Tested compounds and vehicle alone were injected 30 min before the carrageenan injection and the degree of the carrageenan-induced swelling of the paw was measured for 3 h after carrageenan injection using a mercury plethysmograph (Ugo Basil, Italy).
The degree of swelling induced was evaluated by the ratio a/b, where a and b are total volumes of both hindpaws before and after carrageenan treatment respectively. A ratio smaller than 1.5 after drug administration was considered as a significant inhibitory effect of the drugs (8).
Statistical analysis
The data are expressed as mean±SEM. Student t-test followed by Tukey-Kramer multiple comparisons test were used to determine significant differences between groups. p-values less than 0.05 were considered as indicative of significance.
Results
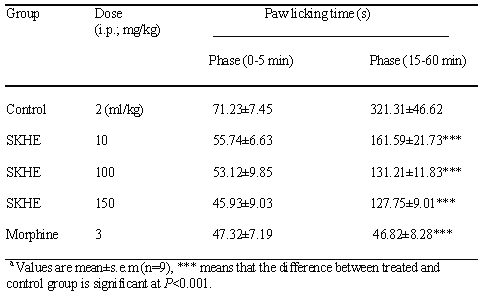
The results of this study for formalin and carrageenan tests are presented in Table 1 and Figure 1 respectively. As displayed in Table 1, no significant difference (p>0.05) is seen between animals treated with SKHE (10-150 mg/kg) and those treated with vehicle in the first phase of formalin test. The same result is seen for morphine sulfate (3 mg/kg) and control group. No significant was seen between morphine (3 mg/kg) and SKHE (10-150 mg/kg).
Table 1: Anti-nociceptive activity of hydroalcoholic extract of S. khuzistanica on formalin test in ratsa.
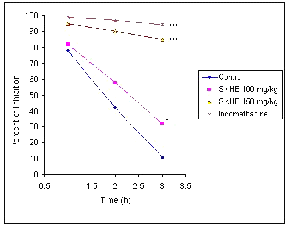
Figure 1: Effect of the S. khuzistanica hydroalcoholic extract (SKHE) on carrageenan-induced edema in rats.
Values are means±s.e.m (n=9); *** P<0.001 compared with control group, * P<0.05 compared with control group.
This table revealed also a significant difference (p <0.001) between all dose of SKHE and morphine with control group in second phase of formalin test. Nevertheless, at the same time there were a significant differences (p <0.001) between morphine and all dose of SKHE in this phase. Different dose effects in formalin test showed a dose-dependent manner over the range of 10-150 mg/kg (y = -0.2531x + 162.12; r 2 = 0.9304) especially in second phase of formalin test.
The result of carrageenan test (Fig 1) showed a significant difference (p<0.001) between both SKHE (150 mg./kg) and indomethacin (4 mg/kg) with control group. These results also revealed the similarity SKHE (150 mg/kg) with indomethacin effect (p> 0.05).
In formalin test, the animals which received SKHE more than 200 mg/kg showed sleepy or some kind of immobility phenomena which interfere with the test.
Discussion
In this study, the anti-nociceptive and anti-inflammatory effects of SKHE were investigated. The formalin test is believed to represent a significant model of clinical pain and formalin produced a distinct biphasic response to pain stimulus and different analgesic compounds may act differently in the early and late phases of this test. The early phase is the result of direct chemical activation of nociceptive primary afferent fibers, while the factors that contribute the late phase are not well defined (9,10). Therefore, this test can be used to clarify the possible mechanisms of anti-nociceptive effect of a test compound (9). Centrally acting drugs such as opioids inhibit both phases equally (11) but, peripherally acting drugs, such as cyclooxygenase inhibitors (aspirin and indomethacin) and corticosteroids only inhibit the late phase (12-14).
The effect of SKHE on the first and second phases of formalin test suggests that its activity may be resulted from its peripheral action when compared with morphine activity in this respect to suggest any mediator merits further investigation.
Among the tests most widely used for the screening of new anti-inflammatory agents is the carrageenan-induced edema in the rat hind paw (14). This edema depends on the participation of kinins and polymorphonuclear leukocytes with their pro-inflammatory factors, including prostaglandins (16, 17). Isolation and purification of different fractions of the hydroalcoholic extract and assaying anti-inflammatory/analgesic activity of each fraction was not goal of this study. Nevertheless, based on the results of this study, we can suggest that the anti-inflammatory effect of SKHE may be attributed to inhibition of prostaglandin release and similar mediators involved in this test (15-17). It may also be related partly to the presence of flavonoids, steroids, essential oil and tannin (3) that have been shown to exert analgesic effects in animal models of nociception (18,19).
Carvacrol is one of the most important components of many species including those belong to Satureja genus (20-23). This phenolic compound has shown antiseptic, antibacterial, antifungal as well as anti-noceceptive and anti-inflammatory properties in Satureja spp. (20,22,24-25).
Furthermore, humoral reactions resulting in the antioxidative action effect and anti-inflammatory activity of major constituents of this species may attribute to the anti-inflammatory effect of this plant. In any case, the consistent of the result of this study with indomethacin effect can take into account to confirm the traditionally use of this plant as an analgesic and anti-inflammatory agent.
Thus, it seems that the extract relieved pain through both central and peripheral mechanisms. Further studies are necessary to elucidate the exact mechanism behind its traditional effects.
Acknowledgements
This study was carried out with financial support from Research Council of Tehran University of Medical Sciences.
References
Sosa S, Balick MJ, Arvigo R, Esposito RG, Pizza C, Al-inier G, Tubaro. Screening of the topical anti-inflammatory activity of some Central American plants. J Ethnopharmacol. 2002, 81: 211-215.
Jamzad Z. A new species of the genus Satureja (Labiatae) from Iran. Iran Journ. Bot. 1994, 6: 215-218.
Farsam H, Amanlou M, Radpour M. R, Salehinia AN, Shafiee A. Composition of the essential oils of wild and cultivated Satureja khuzistanica Jamzad from Iran. Flavour Fragr. J. 2004, 19: 308–310.
Sefidkon F, Ahmadi Sh. Essential oil of Satureja khuzistanica Jamzad. B-02, 30TH International symposium on essential oils (30TH ISEO), Sept. 5–8, 1999, Leipzig.
Abdollahi M, Salehnia A, Mortazavi SH, Ebrahimi M, Shafiee A, Fouladian F, Keshavarz K, Sorouri S, Khorasani R, Kazemi A. Antioxidant, antidiabetic, antihyperlipidemic,reproduction stimulatory properties and safety of essential oil of Satureja Khuzestanica in rat in vivo: a oxicopharmacological study. Med Sci Monit. 2003, 9: BR331-5
Farsam H, Amanlou M, Dehpour AR, Jahaniani F. Anti-inflammatory and analgesic activity of Biebersteinia multifida DC. root extract. J. Ethnopharmacol. 2000, 71: 443-447.
Dubuisson D, Dennis SG. The formalin test: a quantitative study of the analgesic effect of morphine, meperidine and brain stem stimulation in rats and cats. Pain 1977, 4: 167-174.
Niemegeer CJE, Verbruggen FJ, Janssen PAJ. Effect of various drugs on carrageenan-induced oedema in the rat hind paw. J. Pharm. Pharmacol. 1964, 16: 810–816.
Tjolsen A, Berge OG, Hunskaar S, Rosland JH, Hole K. The formalin test: an evaluation of the method. Pain 1992, 51: 5–17.
Abbadie C, Taylor BK, Peterson MA, Basbaum AI. Differential contribution of the two phase of the formalin test to the pattern of c-fos expresion in the rat spinal cord: studies with remifentanil and lidocaine. Pain 1997, 69: 101-110.
Shibata M, Ohkubo T, Takahashi H, Inoki R. Modified formalin test: characteristic biphasic pain response. Pain 1989, 38: 347–352.
Hunskaar S, Hole K. The formalin test in mice: dissociation between inflammatory and non-inflammatory pain. Pain, 1987, 30: 103–114.
Rosland JH, Tjoisen A, Maehle B, Hole K. The formalin test in mice, effect of formalin concentration. Pain, 1990, 42: 235–242.
Chan YF, Tsai HY, Tian-Shang W. Anti-inflamatory and analgesic activities from roots of Angelica pubescens. Planta Medica, 1995, 61: 2–8.
Spector WG. The inflammatory response. J. Path. Bacterol. 1962, 84: 391–403.
Di Rosa M, Giroud JP, Willoughby DA. Studies of the mediators of the acute inflammatory response induced in rat in different site by carrageenan and turpentine. J. Path. 1971, 104: 15–29.
Ferreira SH, Moncada S, Van JR. Some effects of inhibiting endogenuse prostaglandin formation on the responses of the cat spleen. Br. J. Pharm. 1973, 47: 48–58.
Calixto JB, Beirith A, Ferreira J, Santos AR, Cechinel Filho V, Yunes, RA. Naturally occurring anti-nociceptive substances from plants. Phytother Res. 2000, 14: 401-418.
Peres MTLP, Delle Monache F, Pizollatti MG, Santos ARS, Beirith A, Calixto JB, Yunes RA. Analgesic compounds of Croton urucurana Baillon. Pharmaco-chemical criteria used in their isolation. Phytother. Res. 1998, 12: 209-214.
Azaz D, Demirci F, Satil F, Kurkcuoglu M, Baser KHC. Antimicrobial activity of some Satureja essential oils. Z. Naturforsch. 2002, 57c: 817-821.
Akgul A, Ozcan M. Essential oil of four Turkish wild-growing Labiatae herb: Salvia cryotantha Montbr. et Auch., Satureja couneifolia Ten. Thymbra spicata L. and Thymus cilicicus Boiss. Et Bal. J. Essent. Oil. Res. 1999, 2: 209-214.
Kurkcuoglu M, Tumen G, Baser KHC. Essential Oil Constituents of Satureja boissieri from Turkey. Chemistry of Natural Compounds. 2001: 37: 329-331.
USDA, ARS, National Genetic Resources Program, Phytochemical and Ethnobotanical Database. [Online Database] National Germplasm Resources Laboratory, Beltsville, Meryland, 02 Jan. 2005. url: http://sun.ars-grin.gov.
Hajhashemi V, Ghannadi A, Pezeshkian SK. Antinocicieptive and anti-inflammatory effects of Satureja hortensis L. extracts and essential oil. J. Ethnopharmacology 2002, 82: 83-87
Corresponding Author: Hassan Farsam, Department of Medicinal Chemistry, Faculty of Pharmacy, Tehran University of Medical Sciences, Tehran, Iran. farsam@sina.tums.ac.ir
Published by the Canadian Society for Pharmaceutical Sciences.
Copyright © 1998 by the Canadian Society for Pharmaceutical Sciences.
http://www.cspscanada.org