J Pharm Pharmaceut Sci (www.cspscanada.org) 8(1):63-68, 2005
Evaluation of the relaxant action of some Brazilian medicinal plants in isolated guinea-pig ileum and rat duodenum.
Fernanda Emendörfer, Fabiane Emendörfer, Fernanda Bellato, Vânia F. Noldin, Rivaldo Niero, Valdir Cechinel-Filho
Programa de Mestrado em Ciências Farmacêuticas/ Núcleo de Investigações Químico-Farmacêuticas (NIQFAR)- Universidade do Vale do Itajaí - UNIVALI, Itajaí, Santa Catarina, BrazilAlcíbia Maia Cardozo1
Department of Pathology, Universidade Federal de Santa Catarina, Florianópolis, Santa Catarina, BrazilReceived 18 November 2004, Revised 18 January 2005, Accepted 4 February 2005, Published 18 March 2005
PDF Version
Abstract
PURPOSE. The present study aimed to evaluate the possible antispasmodic activity in vitro of methanolic extracts (ME) of six Brazilian medicinal plants. METHODS. The extracts were evaluated on isolated guinea-pig ileum and rat duodenum preparations. RESULTS. Rubus imperialis, Maytenus robusta, Ipomoea pes caprae and Epidendrum mosenii did not inhibit the contractile response elicited by acetylcholine on guinea-pig ileum. On the other hand, ME from Calophyllum brasiliense and Cynara scolymus exhibited significant inhibitory activity for the contractile response elicited by acetylcholine on guinea-pig ileum and on rat duodenum in a noncompetitive and concentration-dependent manner. CONCLUSIONS. The current study suggests that, of the six medicinal plants evaluated, only the ME of Calophyllum brasiliense and Cynara scolymus show probable antispasmodic activity, confirming and justifying their use in folk medicine for the treatment of intestinal disorders.
Introduction
Several plants are used in folk medicine in many countries, including Brazil, for the treatment of intestinal disorders, such as diarrhea, and spasms, among others, because their presumed antispasmodic properties [1-3]. In a program developed in our university with the aim of searching for new antispasmodic agents from natural sources, we selected some Brazilian medicinal plants employed in folk medicine for the treatment of intestinal disturbances or those previously investigated by our research group in other experimental models.
Rubus imperialis (Rosaceae) occurs abundantly in the South of Brazil, being commonly known as "amora-branca", "amora-do-mato" or "amora-brava", and it is frequently used by rural communities to treat diabetes and dolorous processes. The genus Rubus clearly show hypoglycemic activity, antibacterial effects against Gram-positive bacteria and anti-allergic activities against allergic rhinitis, atopic dermatitis and asthma. Some extracts and a compound denominated Niga-ichigoside F1, isolated from Rubus imperialis, exhibit interesting antinociceptive profile [4, 5].
Maytenus robusta (Celastraceae), which is very well adapted to the South of Brazil, is suggested for use in phytotherapic preparations instead of Maytenus ilicifolia ("espinheira-santa"), which is presently near extinction in Brazil [6].
Ipomoea pes caprae (Convolvulaceae) known as "salsa-da-praia", "pé-de-cabra" or "batateira-da-praia" in Brazil, is used in folk medicine against inflammation and gastrointestinal disorder and as an analgesic agent. Our research group demonstrated the analgesic properties for extracts and isolated compounds from this plant [7, 8]. Pongprayoon and co-workers described antispasmodic activity for Ipomoea pes caprae and some of its constituents [9, 10], which was collected in Europe, and exhibited a marked difference in terms of the chemical composition from the plant collected in Brazil [11].
Epidendrum mosenii (Orchidaceae), known as "orquídea-da-praia, is an ornamental plant, which is sometimes used against infectious and dolorous pathologies. The methanolic extract and some triterpenes and steroids obtained from this plant have demonstrated pronounced antinociceptive activity in mice [12, 13].
Calophyllum brasiliense (Guttiferae) popularly called of "guarandi" or "guanandi" is used against inflammatory and dolorous processes and for the treatment of ulcers. Previous studies have confirmed these pharmacological properties [14, 15]. Chromanone acids isolated from Calophyllum brasiliense showed moderate to strong antibacterial activity [16] and the coumarins were cytotoxic against human tumor cell lines in vitro [17].
Cynara scolymus (Compositae), known as "alcachofra" or "artichoke" is used for the treatment of hepatitis, hiperlipoproteinemia and cholesterolemia [18, 19, 20]. The artichoke leaf extract alleviate symptoms and improve the disease-specific quality of life in patients with functional dyspepsia [21]. Artichokes have been demonstrated to be a promising source of phenolics that may be used as natural antioxidants or functional food ingredients [22]. In addition, we have previously reported that this plant exhibits diuretic effects in rats [23].
In this study, the methanolic extracts from the above mentioned Brazilian medicinal plants species were examined for a possible antispasmodic effects against acetylcholine-induced contractile activity on isolated guinea-pig ileum and rat duodenum.
Material and Methods
Plant material
The plant material used in this study was authenticated by Dr Ademir Reis (Department of Botany, UFSC, Florianópolis, Brazil), and a specimen voucher for each plant, except Cynara scolymus, was deposited at the Barbosa Rodrigues Herbarium (Itajaí-SC), indicated below:
Rubus imperialis Chum. Schl. (Rosaceae), leaves, roots and stems was collected in Florianópolis, Brazil, in June 1997, and deposited under number V.C. Filho 012.
Maytenus robusta Reiss (Celastraceae), aerial parts was collected in Morro do Baú Ecological Park, Ilhota, Brazil, in October 1997 and a voucher specimen was deposited under number V.C. Filho 016.
Ipomoea pes caprae (L.) R. Br. (Convolvulaceae) aerial parts were collected from a population growing on the dunes of Jurere beach in Florianópolis, Brazil, in March 1997. The voucher specimen was deposited under number V.C. Filho 009.
Epidendrum mosenii Rchb. F. (Orchidaceae) stems were collected in November 1996 at the Brava beach, Itajaí, Brazil. The voucher was deposited under number V.C. Filho 003.
Calophyllum brasiliense Cambess (Clusiaceae), leaves were collected in the gardens of UFSC, Florianópolis, Brazil, in December 1998. Vouchers were deposited under number V.C. Filho 007.
Cynara scolymus Linné (Compositae) leaves were picked from "Central de Plantas" at Curitiba, in February 2000.
Preparation of methanolic extracts
Leaves, roots and stems of Rubus imperialis, aerial parts of Maytenus robusta, leaves of Calophyllum brasiliense and Cynara scolymus and stems of Epidendrum mosenii were dried for two days at 45-50°C. After this period, the dried parts of these plants above and the fresh aerial parts of Ipomoea pes caprae were cut into small pieces and extracted with methanol at room temperature for a period of approximately ten days. After removing the solvent under reduced pressure, the respective methanolic extracts were obtained.
The methanolic extracts were dissolved in ethanol solution (10%) at the desired concentration, immediately prior to use.
Animals
Hartley guinea-pigs (300-400g) of both sexes and male Wistar rats (250-350g) were kept in automatically controlled temperature conditions (23 ± 2°C), in 12 h light-dark cycles, with food and water "ad libitum". 48 hours prior to the experiments, the animals were kept in the laboratory, and 24 hours prior to the experiment, they were deprived of food. All experimental procedures were approved by the Animal Care and use Committee of UNIVALI University.
Evaluation of pharmacological activity
Guinea-pig ileum
Guinea-pigs of both sexes were sacrificed and the ileum was removed. The terminal portions, of about 10 to 20 mm in length, were taken after discarding the 15 cm portion nearest to the ileum-caecal junction [24]. The intestinal content was eliminated by washing with Tyrode solution and the mesenteric residues were removed. Preparations were set up for recording the isotonic contractions in 5 mL jacketed organ baths containing Tyrode solution at 37°C, continuously bubbled with air under 1g of load by means of a light lever (six fold amplification) recorded in kymograph. The Tyrode solution had the following composition (mM): NaCl 136.8; KCl 2.7; CaCl2 1.3; NaHCO 3 12.0; MgCl 2 0.5; Na 2 PO 4 0.14 and glucose 5.5. After an initial equilibration period of about 30-45 minutes, cumulative concentration-effect curves for acetylcholine (1 pM to 100 mM) in the absence or presence of methanolic extract (ME) (0.10-2.0 mg/mL) incubated for 15 minutes were obtained. Six cumulative concentration-effect curves were obtained for each preparation, with a period of at least 20 minutes between each one. The maximum response obtained from the first cumulative concentration--effect curve was taken as the 100% response value. In separate sets of experiments, in order to correct for spontaneous and-or vehicle-induced desensitization, controlled experiments were performed for acetylcholine, in the presence of corresponding concentrations of vehicle.
Rat duodenum
After discarding the 10 cm nearest to the gastroduodenal junction, the duodenum muscle strips (15 to 20 mm), free from adhering tissues, were removed from Male Wistar rats and set up for recording the isotonic contractions in 5 mL jacketed organ baths containing Tyrode solution at 37°C, continuously bubbled with air under 1g of load [24]. After an equilibrium period of at least 30-45 minutes, cumulative concentration-effect curves for acetylcholine (10 nM to 100 mM), were obtained for the absence or presence of different concentrations of ME (0.10-2.0 mg/mL), incubated for 15 minutes. The maximum response obtained from the first cumulative concentration-effect curve was taken as the 100% response value. In separate sets of experiments, in order to correct for spontaneous and-or vehicle-induced desensitization, controlled experiments were performed for acetylcholine, in the presence of corresponding concentrations of vehicle.
Drugs
The drugs used were: acetylcholine iodide (from the Sigma Chemical Company, St. Louis, USA), stored as 0.1M stock solutions for up to a week at -4°C and diluted to the desired concentrations in 0.9% saline just before use. Glucose, NaCl, KCl, CaCl 2 .2H 2 O, MgCl 2 .6H 2 O, NaHCO 3 and NaH 2 PO 4 was acquired from Merck KGA, Darmstadt, Germany. The vegetal extracts were dissolved in a solution of 10% ethanol. The final bath concentration of ethanol had no effect per se on the tonus of the preparations or on agonist-induced contraction.
Statistical analysis
The data are shown as media ± SEM, except for the IC50 (concentration of extracts causing half maximal responses) which are presented as geometric means accompained by their respective 95% confidence intervals. Statistical analysis were performed by means of the unpaired Student "t" test or by analysis of variance followed by the Tuckey test when appropriate, and P < 0, 05 was considered significant.
Results and Discussion
The incubation of increasing concentrations of ME from Rubus imperialis, Maytenus robusta, Ipomoea pes caprae and Epidendrum mosenii (0.1-2.0 mg/mL) for 15 minutes does not inhibit the contractile response elicited by acetylcholine on guinea-pig ileum (results not shown). These medicinal plants do not affect the contraction induced by acetylcholine in isolated guinea pig ileum with the maximal concentration used, there being no statistical significance between the responses obtained from different extract concentrations.
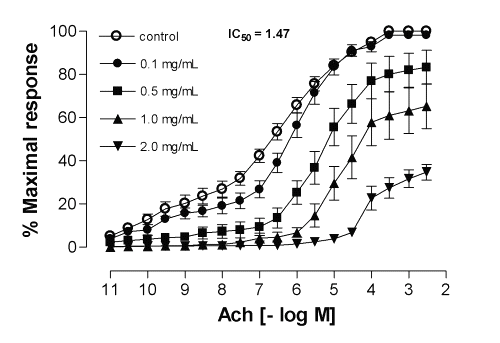
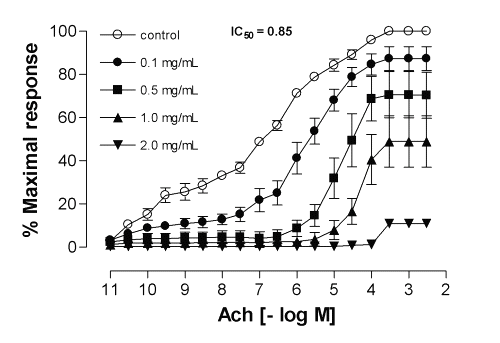
In marked contrast to the results obtained for the four medicinal plants indicated above, the same concentration of ME from Calophyllum brasiliense and Cynara scolymus cause a significant inhibitory effect for the contractile response elicited by acetylcholine on guinea-pig ileum in a noncompetitive and concentration-dependent manner, with IC50 values (with 95% confidence limits, mg/mL) of 1.47 (0.64-3.42) and 0.85 (0.54-1.36), respectively (Fig. 1 and 2). Both methanolic extracts did not interfere with the basal tension of the preparations, indicating that no agonistic effect was present, with restoration of the contractile response to agonist after successive washings.
Figure 1: Mean inhibitory effect of methanolic extract of Calophyllum brasiliense on acetylcholine-induced contractions in the isolated guinea-pig ileum. Each point represents the mean of 6 experiments and the vertical bars represent the S.E.M.
Figure 2: Mean inhibitory effect of methanolic extract of Cynara scolymus on acetylcholine-induced contractions in the isolated guinea-pig ileum. Each point represents the mean of 6 experiments and the vertical bars represent the S.E.M.
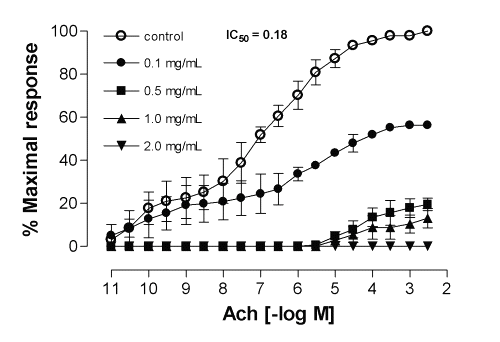
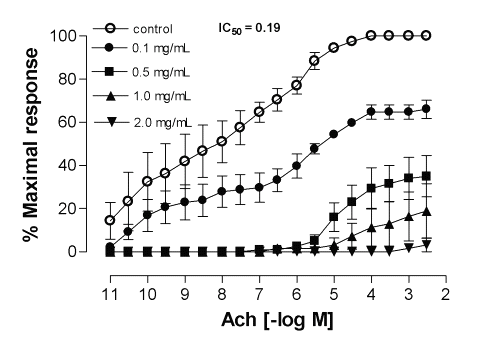
Since only ME from Calophyllum brasiliense and Cynara scolymus exhibited relaxant activity of interest in this experimental model, they were also evaluated against other smooth muscle preparations, from rat duodenum, and the results demonstrated a significant inhibitory effect for the contractile response elicited by acetylcholine in a noncompetitive and concentration-dependent manner, with IC50 values (with 95% confidence limits, mg/mL) of 0.18 (0.15-0.24) and 0.19 (0.02-1.51), respectively (Fig.3 and 4).
Figure 3: Mean inhibitory effect of methanolic extract of Calophyllum brasiliense on acetylcholine-induced contractions in the isolated rat duodenum. Each point represents the mean of 6 experiments and the vertical bars represent the S.E.M.
Figure 4: Mean inhibitory effect of methanolic extract of Cynara scolymus on acetylcholine-induced contractions in the isolated rat duodenum. Each point represents the mean of 6 experiments and the vertical bars represent the S.E.M.
It is interesting to note that in both tissues preparation, a noncompetitive antagonism was observed, characterized by a clear displacement to the right of the concentration-response curves with a decrease in maximal response above 50%. This fact has been also observed with others medicinal plants such as Marrubium vulgare, Droserae Herba and Bacopa monniera [24, 25, 26]. Furthermore, the difference between inhibitory response observed by C. brasiliense and C. scolymus on guinea-pig ileum and rat duodenum was also demonstrated in the same preparations by hydroalcoholic extract of Marrubium vulgare, with IC50 values (mg/mL) of 0.80 and 0.08, respectively [24], which may be due differences of sensibility of each tissue.
The smooth muscle of the guinea-pig ileum undergoes a biphasic mechanical response when exposed to acetylcholine. The first phase, termed the phasic response, consisted of a rapid increase in tension which reached a sharp peak, followed by a rapid reduction in tension, which depended on an initial mobilization of intracellular calcium, probably from the sarcoplasmic reticulum or from calcisomes, in an inositol triphosphate (IP3) dependent way. The second phase, the tonic response, consists of a slower, more sustained increase in tension that is usually of a lesser magnitude. This response is more dependent on calcium from the extracelular medium and is due to an increased calcium influx across the membrane [27].
In this context, the inhibitory effect of ME from Callophylum brasiliense and Cynara scolymus could be caused by a reduction of calcium influx by way of calcium channels and/or through inhibition of calcium release from intracellular stores, decreasing the calcium concentration available for contractile machinery. However, these hypotheses require further studies. To the best of our knowledge, this is the first study to demonstrate the relaxant properties of these plants.
On the other hand, the presence of chemical compounds like coumarins and flavonoids on Calophyllum brasiliense and Cynara scolymus, respectively, should be considerated for the pharmacologycal activity [17, 22]. In this regard, studies with coumarins and flavonoids obtained from different medicinal plants, such as clausmarin-A [28], flavones, flavonols, isoflavones and chalcones [29] and quercetin and isoquercitrin [30], have been reported spasmolytic activity on isolated tissues like guinea-pig trachea and ileum.
In summary, the current study indicated that, of the six medicinal plants evaluated, only two suggested possible antispasmodic activity. Experiments in this area are currently being conducted with Cynara scolymus, the most promising plant evaluated in this study, in order to determine the chemical constituents responsible for the effect described and to elucidate the pharmacological mechanism of action.
Acknowledgements
The authors are grateful to FUNCITEC-SC and CNPq/Brazil by financial support.
References
Orisadipe, A., Amos, S., Adesomoju, A., Binda, L., Emeje, M., Okogun, J., Wambebe, C., Gamaniel, K., Spasmolytic activity of methyl angolensate: a triterpenoid isolated from Etandrophragma angolense. Biol Pharm Bull, 24(4):364-367, 2001.
Weimann, C., Goransson, U., Pongprayoon-Claeson, U., Claeson, P., Bohlin, L., Rimpler, H., Heinrich, M., Spasmolytic effects of Bacharris conferta and some of its constituents. J Pharm Pharmacol, 54:99-104, 2002.
Heinrich, M., Ethnobotany and natural products: the search for new molecules, new treatments of old diseases or a better understanding of indigenous cultures? Curr Top Med Chem, 3(2):141-154, 2003.
Niero, R., Cechinel-Filho, V., Souza, M. M., Montanari, J. L., Yunes, R. A., Monache, F. D., Antinociceptive activity of Niga-ichigoside F1 from Rubus imperialis. J Nat Prod, 62:1145-1146, 1999.
Kanegusuku, M., Benassi, J. C., Pedrosa, R. C., Yunes, R. A., Cechinel-Filho, V., Cardozo, A. H. M., Souza, M. M., Monache, F. D., Niero, R., Cytotoxic, hypoglycemic activity and phytochemical analysis of Rubus imperialis (Rosaceae). Z Naturforsch, 57:272-276, 2002.
Niero, R., Moser, R., Busato, A. C. B., Yunes, R. A., Reis, A., Cechinel-Filho, V., A comparative chemical study of Maytenus ilicifolia Mart. Reis and Maytenus robusta Reiss (Celestraceae). Z Naturforsch, 56:158-161, 2001.
Souza, M. M., Madeira, A. O., Berti, C., Krogh, R., Yunes, R. A., Cechinel-Filho, V., Antinociceptive properties of the methanolic extract obtained from Ipomoea pes-caprae (L.) R. Br. J Ethnopharmacol, 69:85-90, 2000.
Krogh, R., Kroth, R., Berti, C., Madeira, A. O., Souza, M. M., Cechinel-Filho, V., Monache, F. D., Yunes, R. A., Isolation and identification of compounds with antinociceptive action from Ipomoea pes-caprae (L.) R. Br. Pharmazie, 54:464-466, 1999.
Pongprayoon, U., Bohlin, L., Sandberg, F., Wasuwat, S., Inhibitory effect of extract of Ipomoea pes-caprae on guinea-pig ileal smooth muscle. Acta Pharm Nord, 1(1):41-44, 1989.
Pongprayoon, U., Baeckstrom, P., Jacobsson, U., Lindstrom, M., Bohlin, L., Antispasmodic activity of beta-damascenone and E-phytol isolated from Ipomoea pes-caprae. Planta Med , 58 (1):19-21, 1992.
Krogh R. PhD Thesis, Universidade Federal de Santa Catarina, Florianópolis, Brasil, 2001.
Floriani, A. E. O., Ferreira, J., Santos, A. R. S., Monache, F.D., Yunes, R. A., Cechinel-Filho, V., Analgesic compounds from Epidendrum mosenii stems. Pharmazie, 53 (6):426-427, 1998.
Ferreira, J., Floriani, A. E. O., Cechinel-Filho, V., Monache, F. D., Yunes, R.A., Calixto, J. B., Santos, A. R. S., Antinociceptive properties of the methanolic extract and two triterpenes isolated from Epidendrum mosenii stems (Orchidaceae). Life Sci, 66:791- 802, 2000.
Sartori, N.T., Canepelle, D., De Souza Jr, P. T., Martins, D. T., Gastroprotective effect from Calophyllum brasiliense Camb on experimental gastric lesions in rats and mice. J Ethnopharmacol, 67:149-156, 1999.
Silva, K. L., Santos, A. R. S., Mattos, P. E. O., Yunes, R. A., Monache, F. D., Cechinel-Filho, V., Chemical composition and analgesic activity of Calophyllum brasiliense leaves. Therapie, 56:431-434, 2001.
Cottiglia, F., Dhanapal, B., Sticher, O., Heilmann, J., New Chromanone acids with antibacterial activity from Calophyllum brasiliense. J Nat Prod, 67:537-541, 2004.
Reyes-Chilpa, R., Estrada- Muniz, E., Apan, T. R., Amekraz, B., Aumelas, A., Jankowski, C. K., Vazquez-Torres, M., Cytotoxic effects of mammea type coumarins from Calophyllum brasiliense. Life Sci, 75:1635-1647, 2004.
Speroni, E., Cervellati, R., Govoni, P., Guizzardi, S., Renzulli, C., Guerra, M. C., Efficacy of different Cynara scolymus preparations on liver complaints. J Ethnopharmacol, 86(2-3):203-211, 2003.
Englisch, W., Beckers, C., Unkauf, M., Ruepp, M., Zinserling, V., Efficacy of artichoke dry extract in patients with hyperlipoproteinemia. Arzneimittelforschung, 50(3):260-265, 2000.
Gerbhardt, R., Inhibition of cholesterol biosynthesis in primary cultured rat hepatocytes by artichoke (Cynara scolymus L.) extracts. J Pharmacol Exp Ther, 286:1122-1128, 1998.
Holtmann, G., Adam, B., Haag, S., Collet, W., Grunewald, E., Windeck, T., Efficacy of artichoke leaf extract in the treatment of patients with functional dyspepsia: a six-week placebo-controlled, double-blind, multicentre trial. Aliment Pharmacol Ther,18(11-12):1099-1105, 2003.
Schutz, K., Kammerer, D., Carle, R., Schieber, A., Identification and quantification of caffeoylquinic acids and flavonoids from artichoke (Cynara scolymus L.) heads, juice, and pomace by HPLC-DAD-ESI/MS. J Agric Food Chem, 52:4090-4096, 2004.
Noldin, V. F., Cechinel-Filho, V., Monache, F. D., Benassi, J. C., Christmann, I. L., Pedrosa, R. C., Composição química e atividades biológicas das folhas de Cynara scolymus L. (Alacachofra) cultivada no Brasil. Quim Nova, 26 (3):331-334, 2003.
Schlemper, V., Ribas, A., Nicolau, M., Cechinel-Filho, V., Antispasmodic effects of hydroalcoholic extract from Marrubium vulgare on isolated tissues. Phytomedicine, 3:211-216, 1996.
Melzig, M. F., Pertz, H. H., Krenn, L., Anti-inflammatory and spasmolytic activity of extracts from Droserae Herba. Phytomedicine, 8 (3): 225-229, 2001.
Dar, A., Channa, S., Calcium antagonistic activity of Bacopa monniera on vascular and intestinal smooth muscles of rabbit and guinea-pig. J Ethnopharmacol, 66:167-174, 1999.
Gálvez, J., Duarte, J., De Medina, S. F., Jiménez, J., Zarzuelo, A., Inhibitory effects of quercetin on guinea-pig ileum contractions. Phytother Res, 10:66-69, 1996.
Patnaik, G. K., Dhawan, B. N., Evaluation of spasmolytic activity of clausmarin –A a novel coumarin from Clausena pentaphylla (Roxb.) DC. J Ethnopharmacol, 6(2):127-137, 1982.
Ko, W. C., Liu, P. Y., Chen, J. L., Leu, I. J., Shih, C. M., Relaxant effects of flavonoids in isolated guinea pig trachea and their structure-activity relationships. Planta Med, 69 (12):1086-1090, 2003.
Krenn, L., Beyer, G., Pertz, H. H., Karall, E.,Kremser, M., Galambosi, B., Melzig, M. F., In vitro antispasmodic and anti-inflammatory effects of Drosera rotundifolia. Arzneimittelforschung, 54(7), 402-405, 2004.
Corresponding Author: A. M. Cardozo: Departamento de Patologia, Centro de Ciências da Saúde, Universidade Federal de Santa Catarina, Campus Universitário, Trindade, Florianópolis, Santa Catarina, Brazil, CEP: 88040-370. bibamaia@ccs.ufsc.br
Published by the Canadian Society for Pharmaceutical Sciences.
Copyright © 1998 by the Canadian Society for Pharmaceutical Sciences.
http://www.cspscanada.org