J Pharm Pharmaceut Sci (www.cspscanada.org) 7(4):17-21, 2004
Evaluating gelatin based nanoparticles as a carrier system for double stranded oligonucleotides.
Jan Zillies, Conrad Coester1
Department of Pharmacy, Pharmaceutical Technology and Biopharmaceutics, Ludwig-Maximilians-University, Butenandtstraße, Munich, GermanyReceived 15 October 2004, Revised 8 December 2004, Accepted 22 December 2004, Published 02 February 2005
PDF Version
Abstract
PURPOSE: Surface modified gelatin nanoparticles were tested as a potential carrier system for double stranded DNA and RNA oligonucleotides. The results will be discussed with regard to former experiments conducted with single stranded oligonucleotides. METHODS: Gelatin nanoparticles were prepared by a two step desolvation method and surface modified by the covalent coupling of a quaternary amine to obtain a permanent positive net charge. Oligonucleotide loading was conducted in three different media applying 50 mg oligonucleotide per mg nanoparticles in total. Five batches of nanoparticles varying in size and zeta potential (z) were tested. The zeta potentials were determined under enforced ionic conditions in a 10mmol sodium chloride solution at pH 7.0. The separation of unbound oligonucleotides and gelatin nanoparticles was achieved by centrifugation. Free oligonucleotide was determined UV-spectrophotometrically (260nm) in the supernatant. RESULTS: It could be shown that up to 50 mg nucleic acid per mg nanoparticles can be bound depending on the particle's zeta potential and the chosen incubation medium. CONCLUSIONS: The results suggest that the proposed procedure allows a successful drug loading of double stranded oligonucleotides onto to the surface of accordingly modified gelatin nanoparticles.
Introduction
The great advances made in the knowledge of the human genome and the resulting therapeutical chances and strategies (1;2) inevitably prompt the need for an adequate vector system. Bearing in mind the targeted delivery of DNA oligonucleotides or plasmids and the rapid digestion of "naked" nucleic acids occurring under in vivo conditions as well as their poor capacity to diffuse through biological membranes (3), this need becomes apparent. Relating to particular therapeutical scenarios, these vector systems have to meet varying requirements. Thus, one may talk about a custom-made character of such vector systems, which are in general viral or non-viral based globular structures in the nanometer scale. High transfection rates and an effective targeting make retroviruses, adenoviruses, and adeno-associated viruses the most popular (viral) vector systems (4;5). Safety concerns, namely the immunogenic potential of the former pathogens, lead to an emerging interest in non-viral alternatives (3;5) such as cationic polymers (6), liposomes (7), and nanoparticles (8). Although these synthetic carriers are less efficient than their viral counterparts, they exhibit advantageous properties concerning safety, simplicity of preparation and high encapsulation capability (9), resulting in an application in gene therapy and related therapeutic approaches. In addition, nanopaticulate drug carriers based on gelatin offer the advantages of a simple and safe delivery system with decades of experience in the i.v. application of the raw material as plasma expander. Related to these attributes Truong-Le et al. described a nanometersized complex coacervate originating from plasmid DNA and gelatin macromolecules as a non viral alternative in the field of gene delivery and gene therapy respectively (10;11). Contrarily to Truong-Le´s proposed nanospheres the aim of this study is to investigate nanoparticles solely consisting of a solid gelatin core as a potential carrier system for double stranded oligonucleotides.
Materials and Methods
Materials
Gelatin Type A with Bloom 175, 1-Ethyl-3-dimethyl aminopropylcarbodiimide (EDC) and 2-Aminoethyl-trimethylammoniumchlodride hydrochloride (cholamine) were obtained from Sigma-Aldrich (Taufkirchen, Germany). Dulbecco's Phosphate Buffered Saline pH 7.4 was purchased from PAA Laboratories GmbH (Linz, Austria). NF-kB decoy oligonucleotides (22-mer, phosphorothioate backbone, double stranded, MW 14145 Da) were purchased from biomers.net GmbH (Ulm, Germany). siRNA oligonucleotides (21-mer, phosphodiester backbone, double stranded, MW 14826 Da) were kindly donated by Qiagen GmbH (Hilden, Germany).
Preparation and surface modification of gelatin nanoparticles
Gelatin nanoparticles were prepared by a two step desolvation method (12) and surface modified as described previously (13). Briefly, after preparation and purification, the nanoparticles were suspended in highly purified water followed by dissolving cholamine in the resulting suspension. After 5 minutes of stirring, EDC was added to the reaction vessel in order to activate the free carboxyl groups on the surface of the unmodified nanoparticles for the coupling with cholamine. The resulting increase of the zeta potential was determined under enforced ionic conditions in a 10mmol sodium chloride solution at pH 7.0 using a Zetamaster 3000 (Malvern Corp., Worcestershire, UK).
Drug loading
Oligonucleotide loading was conducted in highly purified water, a phosphate buffered saline solution (pH 7.4), and a 0.9% NaCl solution. Four different batches of surface modified gelatin nanoparticles, varying in size and zeta potential, were used: NP 0309-1 (size 182nm, z +2.3mV), NP 009-04 (size 228nm, z +7.8mV), NP 010-04 (size 278nm, z +13.3mV), NP 019-04 (size 219nm, z +17,5mV). 50 mg oligonucleotide per mg nanoparticles was dissolved in 1ml medium. The nanoparticles were then incubated with the oligonucleotides for two hours at 800 rpm and 22°C using a Thermomixer (Eppendorf AG, Hamburg, Germany).
Determination of drug loading efficiency
The amount of unbound oligonucleotide was determined UV spectrophotometrically (260nm, UV1, Thermo Spectronic, Dreieich, Germany) in the supernatant of the reaction media after the separation of the nanoparticles by centrifugation at 14000g (neo lab 16/18, Hermle Labortechnik, Wehingen, Germany).
Results and Discussion
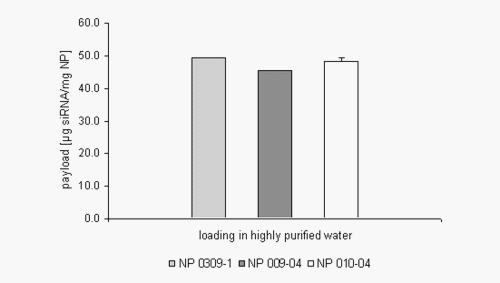
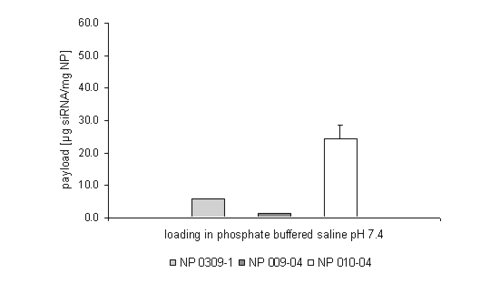
The introduction of quaternary amines like cholamine lead to an increased permanent positive net charge of the gelatin nanoparticles enabling the particles to bind negatively charged drug substances like oligonucleotides onto their surface by ionic interactions. The obtained results show that up to 50 mg of a double stranded siRNA oligonucleotide can be bound per mg gelatin nanoparticles (NP) suspended in highly purified water, which is the standard amount referring to our work with single stranded oligonucleotides. Although this amount seems to be constant for the three tested nanoparticle formulations (Figure 1) it dramatically changed while working in a buffered environment (Figure 2).
Figure 1: siRNA loading efficiency in highly purified water.
Figure 2: siRNA loading efficiency in phosphate buffered saline pH 7.4.
The applied nanoparticle formulations were characterised with an increasing size and zeta potential from the left to the right. When working in PBS buffer, the amount of siRNA loading seems to be much more dependent on these physicochemical properties, especially the zeta potential.
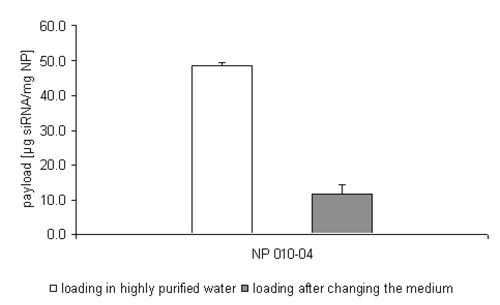
Additionally, some data show, that the replacement of the incubation medium from highly purified water to a phosphate buffered environment during one attempt causes a release of nearly 80% of the formerly bound double stranded oligonucleotide (Figure 3).
Figure 3: siRNA loading efficiency on NP 010-04 after changing the incubation medium to PBS.
In discussing this phenomenon, the ionic strength of the different incubation media has to be taken into account. As already pointed out, the drug loading is based on ionic interactions; the stronger the ionic interactions the more stable the drug loading will be. Therefore, a certain inverse net charge of the nanoparticles and the oligonucleotides is necessary. Working in buffered media with its elevated ionic strength, the zeta potential of the nanoparticles is partially or even completely compensated and therewith also the attractive forces.
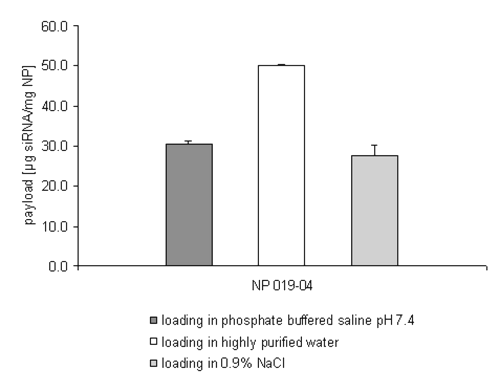
Only the NP 010-04 batch, with a particle size of 278nm and the high zeta potential of +13.3mV has shown a reproducible loading of 25 mg siRNA per mg nanoparticles. To scrutinize whether the zeta potential and not the size of the nanoparticles is in fact the critical parameter, another formulation with a smaller size and a higher zeta potential was evaluated (NP 019-04, size 219nm, z +17.5mV) (Figure 4).
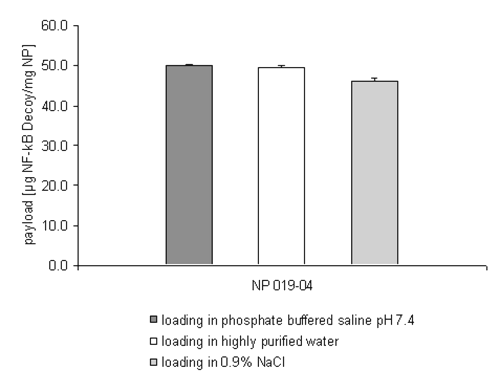
Figure 4: siRNA loading efficiency on NP 019-04 depending on the incubation medium.
The results shown in Figure 4 strengthen the suggestion that the zeta potential and not the particle size is the primary consideration in enabling the drug loading process even under isoosmotic conditions. In comparison to batch NP 010-04 the smaller size of the nanoparticles NP 019-04 doesn't affect the efficiency of the drug loading process. The mass of siRNA bound in PBS buffer reaches roughly the same amount already seen in Figure 2 (NP 010-04).
The drug loading process additionally accomplished in an isoosmotic sodium chloride solution confirms the reduced efficiency of the drug loading process of the double stranded siRNA oligonucleotides in media with an accelerated ionic strength.
It has to be mentioned that even the low zeta potential of the nanoparticles NP 0309-1 (+2.3mV) was adequate to bind up to 50 mg of a single stranded oligonucleotide per mg nanoparticles, even in a PBS buffered environment (14). The flexibility of the single stranded molecule might be a probable explanation for this phenomenon.
Interestingly the discussed disturbing influence of the incubation medium on the drug loading efficiency seems to be negligible when changing the drug substance towards a double stranded NF-kB decoy oligonucleotide (Figure 5).
Figure 5: NF-kB decoy oligonucleotide loading efficiency on NP 019-04 depending on the incubation medium.
In all the tested incubation media, nearly the entire mass of the administered double stranded oligonucleotide was bound onto the surface of the nanoparticles. The molecular structure of this phosphorothioate has to play an important role in explaining this phenomenon. Presumably the substitution of a non-bridging oxygen atom with a sulphur atom in the phosphodiester backbone and the missing hydroxyl group per molecule ribose in the DNA analogous layout lead to an extended flexibility of the whole molecule. This in turn might facilitate the binding of the NF-kB decoy oligonucleotide.
Nevertheless, it is questionable if the diminished oligonucleotide loading efficiency in PBS buffer and sodium chloride solution respectively plays a major role for the in vitro/in vivo application of the formulations. In tissue culture media, as well as in the blood stream after an i.v. injection, the ionic strength is lower and proteins adsorbed onto the nanoparticles may exert even a protective effect on the double stranded oligonucleotide loading. Further studies are under way to evaluate the drug loading in isoosmotic solutions with a reduced ionic strength and in tissue culture media. Moreover, preliminary tests show the successful cellular uptake of NF-kB decoy oligonucleotide loaded gelatin nanoparticles and the desired reduced activity of the targeted transcription factor.
Conclusions
The results show that the drug loading of double stranded oligonucleotides onto the surface of modified gelatin nanoparticles via ionic interactions is feasible. Regarding these results and the advantageous properties of gelatin nanoparticles as highly biocompatible and simple drug delivery vectors, corresponding studies are in progress to further improve the in vitro transfection efficacy.
References
Austin, C. P., The completed human genome: implications for chemical biology. Current Opinion in Chemical Biology, 7:511-515, 2003.
Butcher, S. P., Target discovery and validation in the post-genomic era. Neurochemical Research, 28:367-371, 2003.
Davis, S. S., Biomedical applications of nanotechnology - implications for drug targeting and gene therapy. Trends in Biotechnology, 15:217-224, 1997.
Ferber, D., Gene therapy: safer and virus-free? Science, 294:1638-1642, 2001.
Johnson-Saliba, M. and Jans, D. A., Gene therapy: optimising DNA delivery to the nucleus. Current Drug Targets, 2:371-399, 2001.
Wagner, E., Strategies to improve DNA polyplexes for in vivo gene transfer: will "artificial viruses" be the answer? Pharmaceutical Research, 21:8-14, 2004.
Mhashilkar, A., Chada, S., Roth, J. A. and Ramesh, R., Gene therapy. Therapeutic approaches and implications. Biotechnology Advances, 19:279-297, 2001.
Sabel, B. A., Walz, C. and Ringe, K., Patent: Nanoparticles for DNA administration to a target organ. Germany, 2002-EP8434[2004017945], 30. 29-7-2002. WO.
El Aneed, A., An overview of current delivery systems in cancer gene therapy. Journal of Controlled Release, 94:1-14, 2004.
Truong-Le, V. L., August, J. T., Leong, K. W., Controlled gene delivery by DNA-gelatin nanospheres. Human Gene Therapy, 9:1709-1717, 1998.
Truong-Le, V. L., Walsh, S. M., Schweibert, E., Mao, H. Q., Guggino, W. B., August, J. T., Leong, K. W., Gene transfer by DNA-gelatin nanospheres. Archives of Biochemistry and Biophysics, 361:47-56, 1999.
Coester, C. J., Langer, K., Von Briesen, H. and Kreuter, J., Gelatin nanoparticles by two step desolvation-a new preparation method, surface modifications and cell uptake. Journal of Microencapsulation, 17:187-193, 2000.
Coester, C., Development of a new carrier system for oligonucleotides and plasmids based on gelatin nanoparticles. New Drugs, 1:14-17, 2003.
Fraunhofer, W., Winter, G. and Coester C., Asymmetrical flow field-flow fractionation and multiangle light scattering for analysis of gelatin nanoparticle drug carrier systems. Analytical Chemistry, 76:1909-1920, 2004.
Corresponding Author: Conrad Coester, Department of Pharmacy, Pharmaceutical Technology and Biopharmaceutics, Ludwig-Maximilians-University, Butenandtstraße 5, 81377 Munich, Germany. conrad.coester@cup.uni.muenchen.de
Published by the Canadian Society for Pharmaceutical Sciences.
Copyright © 1998 by the Canadian Society for Pharmaceutical Sciences.
www.cspscanada.org