J Pharm Pharmaceut Sci (www.ualberta.ca/~csps) 7(3):310-314, 2004
Alteration of rat hepatic glycogen phosphorylase and phosphoenolpyruvate carboxykinase activities by Satureja khuzestanica Jamzad essential oil.
Maryam Saadat, Shirin Pournourmohammadi, Mohadeseh Donyavi, Reza Khorasani, Gholamreza Amin, Ali Nazar Salehnia, Mohammad Abdollahi1
Laboratory of Toxicology, Pharmaceutical Sciences Research Centre; and Laboratory of Pharmacognosy, Faculty of Pharmacy, Tehran University of Medical Sciences, IranReceived 17 June 2004, Revised 17 September 2004, Accepted 21 September 2004, Published 24 September 2004
PDF Version
Abstract
PURPOSE: Regarding reported antidiabetic effect of Satureja khuzestanica essential oil (SKEO) and important role of liver on body glucose metabolism by glycogenolysis and gluconeogenesis, we were interested to examine effect of SKEO treatment on rat hepatic key enzymes of glycogenolysis and gluconeogenesis in vivo. METHODS. Effective antidiabetic dose of SKEO (1000 ppm) was administered to rats through drinking water for 2 weeks. After 18 hours fasting post treatment under general anesthesia, the liver was removed by transverse abdominal incision and perfused with cold 0.9% saline and kept frozen at -70șC until homogenized. The activities of the key enzymes glycogen phosphorylase (GP) and phosphoenolpyruvate carboxykinase (PEPKC) were analyzed in the homogenate. Blood sample was taken by cardiac puncture for glucose assay. RESULTS. SKEO treatment did not affect blood glucose concentrations but decreased hepatic PEPCK activity by 26% (P<0.01) of control and increased hepatic GP by 24% (P<0.01) of control. CONCLUSION. Disturbance of hepatic glucose metabolism is proposed as a mechanism of antidiabetic action of SKEO which could be in relation with antioxidant property of this plant.
Introduction
Satureja khuzestanica Jamzad is an endemic plant of Iran that is widely distributed in Southern part of Iran. It is famous for its medical uses as analgesic and antiseptic in folk medicine [1]. The genus Satureja belongs to the family Lamiaceae , subfamily Nepetoideae and the tribe Mentheae . One of the diagnostic characteristics of the subfamily Nepetoideae is that its representatives contain more than 0.5% of essential oil [2]. It has been reported that there are marked differences between and within the subspecies of Satureja essential oil composition [3]. During recent years, antiviral [4], antinociceptive and antiinflammatory [5], antibacterial and antifungal [6-8], antispasmodic and antidiarrhea [9] and vasodilatory [10] effects have been reported for different species of Satureja growing in different parts of world but only one study has been undertaken on Satureja Khuzestanica which published recently [11]. That study indicated antidiabetic effects of Satureja Khuzestanica essential oil (SKEO) when examined in rats in vivo. In blood glucose hemostasis, liver plays a major role that maintains a balance between the uptake and storage of glucose via glycogenesis and the release of glucose via glycogenolysis and gluconeogenesis. Glucose is used continuously at a high rate in mammals by organs such as the brain, red blood cells and renal medulla. The regulation of these metabolic pathways involves the rapid modulation of the activity of specific proteins (enzymes, transporters) and modulating their transcription rate or post-transcriptional steps such as mRNA half-life and translation efficiency [12-14]. Regarding these findings, the aim of this study was to examine whether administration of SKEO might affect activities of hepatic key enzymes of gluconeogenesis and glycogenolysis including phosphoenolpyruvate carboxykinase (PEPCK) and glycogen phosphorylase (GP) in rat.
mATERIALS AND mETHODS
Materials
All chemicals were obtained from Sigma-Aldrich Co. (Dorset, England).
Preparation of essential oil
The aerial parts of plant were collected during the flowering stage of plant from Khoaramabad of Lorestan province. The plant was identified by the Department of Botany of the Research Institute of Forests and Ranglands (TARI), Tehran. A voucher specimen (No. 58416) has been deposited at the Herbarium of TARI. The plant was cultivated in Khoramabad and the aerial parts of the plant were collected during the flowering stage. The aerial parts were air dried at ambient temperature in the shade and hydrodistilled using a Clevenger type apparatus for 5 h, giving yellow oil in 0.9% yield. The oils were dried over anhydrous sodium sulfate and stored at 4 ºC.
Animals
Adult male Wistar rats from animal house of Faculty of Pharmacy, Tehran University of Medical Sciences (Tehran) weighing 180-230 g were used in this study. All animals were housed under standard laboratory conditions and allowed free access to normal laboratory rat chaw and water ad libitum.
Treatment
Animals were randomly divided into two groups comprising of 8 animals and treated for 2 weeks. The treated group of animals received effective antidiabetic dose of SKEO (1000 ppm) dissolved in drinking water [11]. Control group animals received only tap water. At the end of the specified treatment, the liver was removed by transverse abdominal incision and perfused with cold 0.9% saline and kept frozen at -70ºC until homogenized. The weight of liver was recorded. Blood sample was taken under anesthesia by cardiac puncture in vials containing heparin and serum separated and kept at -20ºC for glucose assay.
Analyses
Serum glucose level was measured in the presence of glucose oxidase and peroxidase using o-dianisidine-HCl as a chromogen. The amount of glucose formed is related to amount of o-dianisidine oxidation products that measured spectrophotometrically at 436 nm.
Liver PEPKC activity was measured in the crude extract, briefly after removal, the liver was cut with scissors and homogenized at 4ºC for 2 min with 10 volumes of cold 0.2 M phosphate buffer, pH 7.4, containing 2 mM mercaptoethanol and 2 nM EDTA and the homogenate was centrifuged for 10 min at 60000 g at 0ºC and the supernatant was used for enzyme activity assay. Enzymatic activity was assayed in the reverse direction as described previously [15]. In this method, formation of the oxaloacetic acid in the presence of NADH is assayed. The activity of PEPCK is expressed as unit per gram of liver protein.
To measure liver GP activity, the liver homogenate was centrifuged at 30000 g for 30 min and the supernatant was used for enzyme activity assay. Enzymatic activity was assayed in the direction of glycogen breakdown by measuring the reduction of NADP [16]. GP activity is expressed as unit per gram of liver protein.
Protein content in the supernatant using bovine serum albumin as standard as described previously [17].
Statistical
Values are reported as mean±SE. Statistical significance between groups was computed using Student t-test. P values greater than 0.05 were considered insignificant.
Results
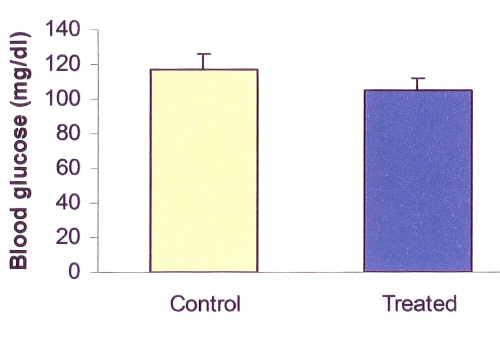
SKEO administration did not significantly change blood glucose concentrations (P>0.05; 105 vs. 117.3; Figure 1).
Figure 1: Effects of Satureja Khuzestanica essential oil (SKEO) on rat blood glucose concentration. SKEO was administered in dose of 1000 ppm for 2 weeks through drinking water. Values are expressed as mean±SE of 8 animals in each group. No significant difference was observed between control and treated groups.
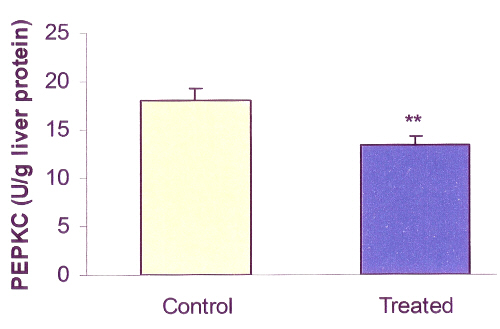
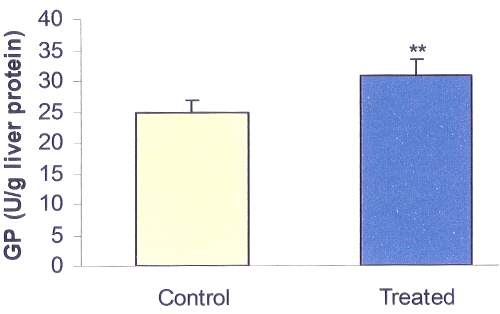
SKEO decreased hepatic PEPCK activity by 26% (P<0.01; 13.4 vs. 18.1; Figure 2) of control and increased hepatic GP by 24% (P<0.01; 31 vs. 25; Figure 3) of control.
Figure 2: Effects of Satureja Khuzestanica essential oil (SKEO) on rat hepatic phosphoenolpyruvate carboxykinase (PEPCK) activity. SKEO was administered in dose of 1000 ppm for 2 weeks through drinking water. Values are expressed as mean±SE of 8 animals in each group. ** Significantly different from control at P<0.01.
Figure 3: Effects of Satureja Khuzestanica essential oil (SKEO) on rat hepatic glycogen phosphorylase (GP) activity. SKEO was administered in dose of 1000 ppm for 2 weeks through drinking water. Values are expressed as mean±SE of 8 animals in each group. ** Significantly different from control at P<0.01.
Discussion
The present study showed that administration of SKEO to rats for two weeks cause a disturbance in metabolism of glucose in the liver by stimulating glycogenolysis and inhibiting gluconeogenesis. Disturbed glycogen metabolism and glucose transport has been suggested as a cause of insulin resistance in patients with diabetes which could be a primary or acquired defect in the pathogenesis of diabetes [18]. The PEPCK is the rate-limiting enzyme of gluconeogenesis and enhanced expression of the PEPCK gene in liver is present in most models of diabetes, and is thought to contribute to the increased hepatic glucose output seen in diabetes [19]. Thus any medicine with potential to alter hepatic gluconeogenesis or glycogenolysis might have significant influence on glucose hemostasis. Our recent study indicated that in vivo treatment of rats by SKEO could significantly attenuate blood lipid peroxidation and simultaneously increase blood total antioxidant power confirming its antioxidant properties. In addition, that study confirmed antidiabetic potential of SKEO treatment in streptozocin-induced diabetic rats [11]. There are evidences indicating that carvacrol and flavonoids are main constituents of Satureja relative species [7, 9, 20, 21]. Supporting these studies, p-cymene and carvacrol have been found as main constituents of the Iranian SKEO [22]. Flavonoids are a class of plant phenolics with significant antioxidant and chelating properties. Their cardioprotective effects stem from the ability to inhibit lipid peroxidation, chelate redox-active metals, and attenuate other processes involving reactive oxygen species. In addition, carvacrol has been found to have significant antioxidant properties [23-25]. The green tea flavonoid, epigallocatechin gallate was reported to have blood glucose-lowering effects in animals with reduction of phosphoenolpyruvate carboxykinase gene expression. Generally, plants containing flavonoids have been known to have potential to treat diabetes in human [26]. It has been reported that troglitazone inhibits expression of the PEPCK gene in isolated hepatocytes by an antioxidant property due to existence of the alpha-tocopherol (vitamin E) moiety in its chemical structure [19]. In this regard, a role for reactive oxygen intermediates in regulation of hepatic glucose production has been reported [27]. There is also evidence that the antidiabetic drug metformin which acts through inhibition of hepatic gluconeogenesis produces concurrent antioxidant effects that are most benefits in treatment of diabetes [28]. Considering the ability of SKEO to reduce hepatic PEPCK activity, with note to above statements, the first mechanism that may come to mind is its antioxidant properties.
The second important enzyme of the liver and the key enzyme of the glycogenolysis is GP. Effects of flavonoids or antioxidants on hepatic GP are not well understood. Both inhibitory and stimulatory effects on hepatic GP activity by (-) - and (+)-catechin as a flavonoid have been reported [29]. In support of the present results on increased GP by SKEO, it has been recently published that isoliquiritigenin a flavonoid found in liquorice roots, increases glycogenolysis in rat hepatocytes perfusion [30].
Our recent study has confirmed that disturbance of gluconeogenesis and glycogenolysis could have significant influences on glucose metabolism and pathogenesis of diabetes [31, 32]. It is concluded that SKEO stimulates glycogenolysis and thus depletes hepatic glycogen storage which in turn needs to be compensated by blood glucose because hepatic gluconeogenesis pathway is occluded and this may be a mechanism for antidiabetic effect of SKEO and seems to be in relation with antioxidant properties of this plant. Additional studies are required to determine whether other S. Khuzestanica distillates have a similar effect.
Acknowledgements
This study was partly supported partly by a grant from Research Council of Tehran University of Medical Sciences.
References
Zargari A: Medicinal Plants. 4th ed. Tehran University Publications, Tehran, 1990, pp. 42–45.
El-Gazzar A, Watson L: A taxonomic study of Labiatae and related genera. New Phytologist, 1970; 69: 451-486.
Slavkovska V, Jancic R, Bojovic S, Milosavljevic S, Djokovic D: Variability of essential oils of Satureja montana L. and Satureja kitaibelii wierzb. ex Heuff. from the central part of the balkan peninsula. Phytochemistry, 2001; 57: 71-76.
Abad MJ, Bermejo P, Gonzales E, Iglesias I, Irurzun A, Carrasco L: Antiviral activity of Bolivian plant extracts. Gen Pharmacol, 1999; 32: 499-503.
Hajhashemi V, Ghannadi A, Pezeshkian SK: Antinociceptive and anti-inflammatory effects of Satureja hortensis L. extracts and essential oil. J Ethnopharmacol, 2002; 82: 83-87.
Yamasaki K, Nakano M, Kawahata T, Mori H, Otake T, Ueba N, Oishi I, Inami R, Yamane M, Nakamura M, Murata H, Nakanishi T: Anti-HIV-1 activity of herbs in Labiatae. Biol Pharm Bull 1998; 21: 829-833.
Azaz D, Demirci F, Satil F, Kurkcuoglu M, Baser KH: Antimicrobial activity of some Satureja essential oils. Z Naturforsch, 2002; 57: 817-821.
Sokovic M, Tzakou O, Pitarokili D, Couladis M: Antifungal activities of selected aromatic plants growing wild in Greece. Nahrung, 2002; 46: 317-320.
Hajhashemi V, Sadraei H, Ghannadi AR, Mohseni M: Antispasmodic and anti-diarrhoeal effect of Satureja hortensis L. essential oil. J Ethnopharmacol 2000; 71: 187–192.
Sanchez de Rojas V, Somoza B, Ortega T, Villar AM, Tejerina T: Vasodilatory effect in rat aorta of eriodictyol obtained from Satureja obovata. Planta Med, 1999; 65: 234-238.
Abdollahi M, Salehnia A, Mortazavi SH, Ebrahimi M, Shafiee A, Fouladian F, Keshavarz K, Sorouri S, Khorasani R, Kazemi A: Antioxidant, antidiabetic, antihyperlipidemic, reproduction stimulatory properties and safety of essential oil of Satureja Khuzestanica in rat in vivo: a toxicopharmacological study. Med Sci Monit, 2003; 9: BR331-BR335.
Hers HG. Mechanisms of blood glucose homeostasis. J Inherit Metab Dis, 1999; 13: 395-410.
Nordlie RC, Foster JD, Lange AJ: Regulation of glucose production by the liver. Annu Rev Nutr, 1999; 19: 379-406.
Abdollahi M, Soleimani F, Kangarlou S: A review on blood glucose variations and affecting parameters. Mid East Pharm, 2003; 11: 6-10.
Chang HC, Lane MD: The enzymatic carboxylation of phosphoenolpyruvate. II. Purification and properties of liver mitochondrial phosphoenolpyruvate carboxykinase. J Biol Chem, 1966; 241: 2413-2420.
Lowry OH, Schulz DW, Passonneau JV: The kinetics of glycogen phosphorylases from brain and muscle. J Biol Chem, 1967; 242: 271-80.
Lowry OH, Roserbrough NJ, Farr AL, Randell RJ: Protein measurement with the folin phenol reagent. J Biol Chem, 1951; 193: 265-275.
Rothman DL, Magnusson I, Cline G, Gerard D, Kahn CR, Shulman RG, Shulman GI: Decreased muscle glucose transport/phosphorylation is an early defect in the pathogenesis of non-insulin-dependent diabetes mellitus. Proc Natl Acad Sci USA, 1995; 92: 983-987.
Davies GF, Khandelwal RL, Wu L, Juurlink BH, Roesler WJ: Inhibition of phosphoenolpyruvate carboxykinase (PEPCK) gene expression by troglitazone: a peroxisome proliferator-activated receptor-gamma (PPARgamma)-independent, antioxidant-related mechanism. Biochem Pharmacol, 2001; 62: 1071-1079.
Sanchez de Rojas VR, Somoza B, Ortega T, Villar AM: Isolation of vasodilatory active flavonoids from the traditional remedy Satureja obovata. Planta Med, 1996; 62: 272-274.
Exarchou V, Nenadis N, Tsimidou M, Gerothanassis IP, Troganis A, Boskou D: 2002. Antioxidant activities and phenolic composition of extracts from Greek oregano, Greek sage, and summer savory. J Agric Food Chem, 2002; 50: 5294-5299.
Sefidkon, F, Ahmadi Sh: Essential oil of Satureja Khhuzistanica Jamzad. J Ess Oil Res, 2000; 12: 427–428.
Burits M, Bucar F: Antioxidant activity of Nigella sativa essential oil. Phytother Res, 2000; 14: 323-328.
Lambert RJ, Skandamis PN, Coote PJ, Nychas GJ: A study of the minimum inhibitory concentration and mode of action of oregano essential oil, thymol and carvacrol. J Appl Microbiol, 2001; 91: 453-462.
Vardar-Unlu G, Candan F, Sokmen A, Daferera D, Polissiou M, Sokmen M, Donmez E, Tepe B: Antimicrobial and antioxidant activity of the essential oil and methanol extracts of Thymus pectinatus Fisch. et Mey. Var. pectinatus (Lamiaceae). J Agric Food Chem, 2003; 51: 63-67.
Waltner-Law ME, Wang XL, Law BK, Hall RK, Nawano M, Granner DK: Epigallocatechin gallate, a constituent of green tea, represses hepatic glucose production. J Biol Chem, 2002; 277: 34933-34940.
Ceppi ED, Titheradge MA: The importance of nitric oxide in the cytokine-induced inhibition of glucose formation by cultured hepatocytes incubated with insulin, dexamethasone, and glucagon. Arch Biochem Biophys, 1998; 349: 167-174.
Cosic V, Antic S, Pesic M, Jovanovic O, Kundalic S, Djordjevic VB: Monotherapy with metformin: does it improve hypoxia in type 2 diabetic patients? Clin Chem Lab Med, 2001; 39: 818-821.
Nyfeler F, Moser UK, Walter P:. 1983. Stereospecific effects of (+)- and (-)-catechin on glycogen metabolism in isolated rat hepatocytes. Biochim Biophys Acta, 763: 50-57.
Abdollahi M, Chan TS, Subrahmanyam V, O'Brien PJ: Effects of phosphodiesterase 3,4,5 inhibitors on hepatocyte cAMP levels, glycogenolysis, gluconeogenesis and susceptibility to a mitochondrial toxin. Mol Cell Biochem, 2003; 252: 205-211.
Atefi M, Ghazanfari S, Ostad SN, Ghahremani MH, Abdollahi M: Alteration of glucose homeostasis by rolipram and milrinone but not sildenafil in rat primary hepatocytes culture. Prog Med Res, 2004; 2(13): 1-12.
Abdollahi M, Donyavi M, Pournourmohammadi S, Saadat M: Hyperglycemia associated with increased hepatic glycogen phosphorylase and phosphoenolpyruvate carboxykinase in rats following subchronic exposure to malathion. Comp Biochem Physiol C Toxicol Pharmacol, 2004; 137: 343–347.
Corresponding Author: Mohammad Abdollahi, Department of Toxicology & Pharmacology, Faculty of Pharmacy, and Laboratory of Toxicology, Pharmaceutical Sciences Research Center, Tehran University of Medical Sciences, Tehran 14155-6451, Iran. mohammad.abdollahi@utoronto.ca
Published by the Canadian Society for Pharmaceutical Sciences.
Copyright © 1998 by the Canadian Society for Pharmaceutical Sciences.
http://www.ualberta.ca/~csps