J Pharm Pharmaceut Sci (www.ualberta.ca/~csps) 7(3):359-371, 2004
Influence of the pro-inflammatory cytokines on P-glycoprotein expression and functionality.
Christine Fernandez1, Marion Buyse, Michèle German-Fattal, François Gimenez
Faculté de Pharmacie, Université Paris XI, Pharmacie clinique, Châtenay-Malabry, France; Université Paris XI, CNRS UMR, Le Plessis-Robinson, FranceReceived 31 May 2004, Revised 29 Octob er 2004, Accepted 4 November 2004, Published 17 November 2004
PDF Version
Abstract
PURPOSE: P-glycoprotein (P-gp) is involved in the transport of many drugs at different barriers with consequence in terms of drug distribution and elimination. The expression and activity of P-gp can be modulated by different factors and pathologies. The present article reviews the knowledge regarding the effect of pro-inflammatory cytokines (TNFa, IL-1b, IL-6, IL-2, IFNγ) on the expression and the functionality of P-gp at three major sites of drug absorption and disposition: the liver, the blood-brain barrier, and the intestine. METHODS: The various methods used to study the effect of pro-inflammatory cytokines include in vivo models (i.e. animals infected with Staphylococcus sp, animals injected with bacterial lipopolysaccharide or directly with cytokines, ...) and in vitro models (i.e. primary rat hepatocytes, human brain endothelial cells, ...). RESULTS: The data on P-gp expression and/or function may differ according to the compound used to induce inflammation. However, there is a general trend towards a decrease in both the expression of P-gp (mRNA and protein) and its functionality. Transcription factors and nuclear receptors are probably involved in this regulation. CONCLUSION: Cytokines may interfere with P-gp. Hence, in pathological conditions (inflammation, infection, ...), the expression and functionality of P-glycoprotein may be modulated with consequences for drug disposition and, consequently treatment efficacy.
Introduction
P-glycoprotein (P-gp) is an ATP-dependent efflux transporter, first identified in drug-resistant Chinese hamster ovary cells (1). Its over expression is at the origin of the multidrug resistance (MDR) phenomenon occurring during tumour chemotherapy. P-gp is encoded by mdr 1a, 1b and 2 genes in rodents and MDR 1 and 2 genes in humans. This protein is able to extrude drugs from cells, leading to a decrease in drug concentration within the cell and reduced cancer-chemotherapy efficacy. P-gp is also present in normal tissues where it protects the organism from many drugs by decreasing intracellular concentrations (2). Liver, intestine and brain barriers were the most studied P-gp expressing barriers. Changes in the expression of efflux transporters at these barriers may have consequences on drug disposition and, therefore, on clinical efficiency.
P-gp functionality, protein expression and mRNA expression are influenced by a number of xenobiotics (3) or endogenous compounds (4). P-gp function may be inhibited by many drugs, either used in therapeutics (verapamil, cyclosporin A, quinidine,...) or specifically developed by pharmaceutical companies (valspodar, elacridar,...) (3). Conversely, its activity may be induced in human by drugs like rifampin (5). Pathological conditions, such as infections or inflammation, also may alter the function of the barriers that express P-gp. During infection and/or inflammation process, pro-inflammatory cytokines are released and may affect the initiation of coagulation, the traffic of polymorphonuclear cells to the site of inflammation and the regulation of other cytokines. Inflammatory cytokines may also be responsible for alterations in protein synthesis. Indeed, albumin synthesis is upregulated during inflammation. Other proteins like P-gp might be regulated by pro-inflammatory cytokines.
Since it has been demonstrated that the efficacy of tumour treatment is increased by combining conventional chemotherapeutic drugs with cytokines (6), it appeared of great interest to investigate the interactions between the MDR phenomenon and cytokines and to investigate how cytokines could modulate mdr expression. Various models have been used to study the influence of cytokines on P-gp expression and function, either by assessing the direct effect of isolated cytokines injected to animals or added to cell-culture media, or by inducing pathological conditions with cytokine release such as inflammations, with assessment of P-gp expression and functionality (7).
The aim of the present paper is to focus on the regulation of the expression and the functionality of the efflux protein, P-glycoprotein, by pro-inflammatory cytokines (TNFa, IL-1b, IL-6, IL-2, IFNγ) at hepatic, cerebral and intestinal levels.
This paper reviews what is known about the influence of the cytokines released during acute inflammation and/or infection on P-gp function. We first briefly summarize the data on P-gp structure, distribution and function and on pro-inflammatory cytokines. We then discuss the effects of cytokines on P-gp at three major sites involved in drug disposition: the liver, the blood-brain barrier and the intestine.
P-glycoprotein
Structure and tissue distribution
P-gp is a 170 kD transmembrane phosphoglycoprotein from the ATP-binding Cassette superfamily (ABC), encoded by mdr1a and mdr1b genes in rodents and MDR1 gene in humans and involved in drug transport. The genes mdr2 in rodents and MDR2 in humans play a major role in the transport of phospholipids through phosphatidylcholine translocation into bile (8).
The expression of mRNA or protein may depend on the model used. For example, mdr1b is not detectable in vivo in the blood-brain barrier (9), whereas it can be expressed by cell cultures (10).
P-gp is an efflux pump, which transports a wide range of compounds, preferentially hydrophobic neutral cationic compounds, from the inside of the cell back to the extracellular space. It is constitutively expressed in circulating lymphocytes, epithelial cells of organs with excretory function (intestine, kidney, liver) and in endothelial cells in the brain and placenta. In the small and large intestine, P-gp is expressed on the brush-border membrane of enterocytes. In the liver, it is expressed on the canalicular membrane of hepatocytes (11). P-gp is also located in the luminal membrane of the endothelial cells. Its localisation in brain capillaries is controversial. Some authors evidenced P-gp on astrocytic endfeet (12) and in microglia (13), while it is currently admitted it is mainly localised in endothelial cells. In the central nervous system (CNS), the presence of P-gp has been reported in the epithelium of the choroid plexuses (14). P-gp is also expressed in the capillary endothelial cells of testis.
The role of P-glycoprotein
The main role of P-glycoprotein is to protect against xenobiotics and endogenous compounds at the site of P-gp expression.
The distribution of P-gp suggests that its major role is to excrete xenobiotics and metabolites into urine and bile, and into the intestinal lumen (15). Many drugs are co-substrates or modulators for both systems [P-gp and cytochrome P450 (CYP)]. In the intestine and in the liver, P-gp decreases drug bioavailability by reducing absorption and enhancing the metabolism by CYP enzymes. By producing repeated cycles of absorption and secretion into the intestine, P-gp can increase drug exposure to intestinal CYP and, therefore, can increase drug metabolism (16).
P-gp also prevents drug accumulation and toxicity in the CNS (17). The role of P-gp in drug-CNS disposition was examined using various cell-culture models or homozygously deficient mice for the mdr1a and 1b genes (9). It has been hypothesized that P-gp provides protection against xenobiotics by pumping its substrates from cells to blood. In the brain, P-gp plays a role in the drug transport through the blood-cerebral spinal fluid and the blood-brain barriers. It prevents cerebral accumulation of drugs such as digoxin (18) and central toxicity of compounds such as ivermectin (19). Last, in tumor cells, P-gp transports drugs out of the cells and contributes to the multidrug resistance phenomenon (MDR) (20).
Cytokines
Cytokines are a large group of polypeptides produced by a wide variety of cells (21). T lymphocytes are a major source of cytokine production. Upon activation, they differentiate into two types of helper (Th) cells, according to their cytokine secretion profiles:
- Th1 cells which produce pro-inflammatory cytokines like Tumor Necrosis Factor-a (TNFa), interleukin-1 (IL-1b), interferon gamma (IFNγ), IL-2.
- Th2 cells, which preferentially secrete IL-4, IL-5, IL-6, IL-10, IL-13.
Inflammation develops when Th1 cells are activated whereas Th2 cells play a role in down-regulating Th1 pro-inflammatory response but also in the control of hypersensitivity. The Th1/Th2 distinction has been enlarged in type 1 and type 2 cytokine classification.
TNFa, IL-1b and IL-6 are mainly released by monocytes or macrophages upon stimulation, although TNFa and IL-6 are also produced by Th1 and Th2 cells, respectively.
TNFa is produced as a soluble trimeric form or as a transmembrane form at the surface of macrophages and activated T cells. It plays a major role in inflammation by promoting extravasation of neutrophils, lymphocytes and monocytes and local adhesion to endothelial cells. It also controls the immune response by modulating T cell activation and inducing cytokine synthesis.
Following bacteria or immunoglobulin ligation to monocyte/macrophage, IL-1b can be released into a local environment where it affects capillary endothelial cells, which secrete chemokines and upregulate the expression of vascular adhesion molecules. This process facilitates mononuclear infiltration into a site of early inflammation. IL-1b also induces its own expression in newly arriving monocytes, thus reinforcing the overall process. In terms of other pro-inflammatory molecules, IL-1b would be necessary for the efficient production of IFN-γ. In fact, IL-1b and TNF-a are generally thought of as prototypical pro-inflammatory cytokines. Of note, IL-1b is an extracellular molecular form of IL-1, whereas IL-1b is secreted. They display 30% of homology.
IL-6 is involved in the stimulation of immunoglobulin secretion and T cell activation. IFNγ is produced by Th1 and NK cells and is involved in the activation of macrophage to induce MHC antigen up-regulation, leukocyte adhesion and the release of proinflammatory cytokines, such as TNFa and IL-1b .
Many studies have demonstrated that cytokines modulate the expression and functionality of proteins. For example, the expression of the cytochrome (CYP) is down-regulated by TNFa in human hepatocytes (22), and by IL-1b and IL-6 in rat hepatocytes (23), thus decreasing CYP-associated metabolism.
Models for the study of cytokine effects on P-gp
Various models can be used to investigate the effects of cytokines on P-gp expression and function. In vitro models of cells from different organs of interest and over-expressing P-gp, let in contact with different concentrations of cytokines over various periods, may be used. Animal models may also be chosen, either by injecting different concentrations of separated cytokines over different periods, or by generating an inflammation process where cytokines are over-released. All these models are complementary and do not always give similar results.
In animals, inflammation can be produced, either by indirect production of cytokines following (i) infection with Staphylococcus sp, E. coli, (ii) injection of bacterial lipopolysaccharide (LPS) (= endotoxin, a component of the cell wall of gram-negative bacteria, which imposes a sepsis-type of inflammation) or turpentine (chemical irritant, provoking an aseptic inflammatory response), or by (iii) direct intravenous or intracerebroventricular (icv) cytokine injection.
Among these models, the indirect production of cytokines following injections of LPS or turpentine are the most commonly used to study the influence of cytokines on the expression and/or functionality of P-gp. However, the type of inflammation and of cytokines produced may vary according to the pro-inflammatory compound injected, the dose and the point of injection. For example, the injection of turpentine produces an acute inflammation that is more localized than the one observed after LPS injection. LPS injection induces IL-1b, IL-2, IL-6, TNFa and interferon γ (IFNγ) release, whereas turpentine only induces IL-1b and IL-6 release. The amount of LPS injected may also account for cytokine released. Furthermore, the effects of LPS on cytokine release also depend on the organ studied. After intraperitoneal injection of LPS, IL-1b and TNFa, Turrin et al showed that mRNAs were upregulated in several brain regions (cortex, cerebellum, hippocampus), spleen, liver and adipose tissue, and that TNFa plasma levels were increased (24). After icv administration of LPS to rats, de Simoni et al have demonstrated the induction of inflammatory cytokine production (IL-6, IL-1b , TNFa ), not only in the brain tissue but also in the peripheric nervous system. Furthermore, IFN γ potentiates LPS action by modulating IL-6 and TNFa expression, but not IL-1b expression and production, whereas IFNγ administered alone does not induce cytokine synthesis (25).
Finally, as turpentine requires more time to induce an inflammatory response, the variations observed in mdr mRNA or protein expression may also reflect differences in the time course of cytokine induction between the two models.
All these points and differences must be considered when driving conclusions from a unique model, regarding an interaction between cytokines and P-gp.
Influence of cytokines on hepatic P-gp
Several factors affect mdr expression in hepatic cells. mdr1b is the less abundant form in normal liver but it is largely sensitive to induction factors, whereas mdr1a and mdr2 are constitutively expressed but inducible at a lesser extent (26). Factors inducing hepatocyte proliferation, such as partial hepatectomy or addition of hepatotrophic growth factors to cell-cultures, increase mdr mRNA levels (27).
Effects of inflammation models on hepatic P-gp
After icv administration to rats of LPS from E. coli , P-gp function and expression were evaluated (28). It was observed that icv injection of LPS have consequences at the hepatic level. Hepatic mdr1a mRNA was down-regulated by 70% at 6 h and returned to the pre-treatment level by 24 or 48 h. By contrast, mdr1b mRNA expression was induced at 6 h and returned to control level at 24 h following LPS treatment. This LPS-induced inflammation, which is initiated at the CNS level, also altered the hepatic elimination of digoxin, a well known P-gp substrate, since the biliary elimination of digoxin was decreased in LPS- versus control-rats. The authors reported a decrease in both the expression of mdr1a mRNA and the function of P-gp, and an increase in mdr1b mRNA. These results suggest that mdr1b does not contribute to the biliary elimination of digoxin.
By 6 h after injection of Klebsiella pneumoniae -LPS in rats, mdr1a expression was decreased and returned to control level by 24 h (29). On the contrary, mdr1b levels were increased by 6 or 24 h after injection of the endotoxin.
Vos et al administered E. coli -LPS to rats and investigated the expression of P-gp both at the level of the immunoreactive protein and of the mRNAs in membrane fractions of whole liver and in isolated hepatocytes (30). A significant increase in mdr1b mRNA was observed from 6 h until at least 48 h after endotoxin administration, whereas the levels of mdr1a and mdr2 remained unchanged. On western blot, the global signal obtained with the P-gp C219 antibody was decreased.
In a model of local inflammation induced in rats injected subcutaneously (sc) with turpentine at 0 and 24 h, the mRNA expression and the transcription rate of mdr1a and mdr1b genes were analysed by RT-PCR and nuclear run-on analysis, respectively (31). Relative to controls, mdr1a and mdr1b mRNAs were reduced by 74 and 68%, respectively, in livers. Transcription rates of these genes were also reduced by 52 and 35%, respectively, indicating that the reduction in mdr levels is mediated through a decrease in gene transcription.
After induction of inflammation in rats with either turpentine (sc) or LPS (ip) injection, both the expression and the functionality of P-gp were depressed (32). Again, 48 h after induction of inflammation with either turpentine or LPS, the hepatic expression of the MDR gene products (protein) was reduced. mdr1a and mdr1b mRNAs were also decreased by 40 to 70%, as compared to controls. Finally, the functionality, estimated by the efflux of the substrate Rhodamine 123, was also depressed by 45 to 65%.
Hartmann et al have compared the influence of cytokine-producing LPS and turpentine inflammatory models and of the injection of separated cytokines (TNFaa , IL-1b and IL-6) on the expression of P-gp in mouse liver (33). Both turpentine, which preferentially produces IL-1b and IL-6 release, and LPS, which induces IL-1b, IL-2, IL-6, TNFa and IFNγ secretion, down-regulated hepatic P-gp, both at the mRNA and protein levels. As concerns, separated cytokines, mice treated with IL-6, but not IL-1b and TNFa, displayed significant decreases in the levels of the protein P-gp and of mdr1a, mdr1b and mdr2 mRNAs, which were similar to that observed with experimentally-induced inflammation with turpentine or LPS. From these results, the authors conclude that, since turpentine-induced inflammation is not associated with TNF α induction, IL-1b and more likely IL-6, rather than TNFa, may be involved in the inflammation-induced down-regulation of P-gp.
After ip injection of LPS from S. typhimurium to rats, no change was observed in the expression of the immunoreactive P-gp (34).
Effects of TNFα alone on hepatic P-gp
By using primary rat hepatocytes cultured with or without TNFa, Hirsch-Ernst et al showed that TNF α , given 4000 IU/ml over 3 days induce mdr1b mRNA and protein (P-gp) expression (35). They also demonstrated that TNFa could increase P-gp functionality as rhodamine 123 intracellular accumulations was decreased by cell pre-treatment with TNFa. Conversely, mdr2 expression was not modified by exogenous TNFa. As mdr1b mRNA is predominantly expressed in hepatocyte culture, the observed TNFα-induced enhance in P-gp expression was mainly due to an increase in mdr1b expression.
In order to investigate the specific role of TNFa in the inflammation induced by Klebsiella pneumoniae endotoxin and its effect on P-gp in the liver, rats were pre-treated with pentoxifylline, an inhibitor of TNFa production. Rats were injected i.p. with both the endotoxin and pentoxifylline, and the biliary excretion of the P-gp substrate, rhodamine, was studied (29). In endotoxin-treated rats, an increase in TNFa plasma levels, a decrease in hepatic mdr1a mRNA expression (6 h after endotoxin injection and a return to control levels 24 hours after) and an increase in mdr1b (6 and 24 h after treatment) were observed. The authors also demonstrated that endotoxin decreased biliary rhodamine 123 clearances, which was unchanged in case of pre-treatment with the TNFa inhibitor, pentoxifylline. These results suggest that the decrease in P-gp functionality and in mdr1a expression is likely due to increased plasma levels of TNFa following endotoxin injection.
In mice treated ip with TNF α (1,000-25,000 IU), the levels of P-gp were not altered (33). Regarding the corresponding genes, mdr1b was significantly increased at high doses of TNFα only (25,000 IU), whereas mdr1a was not significantly altered.
Effects of IL-6 alone on hepatic P-gp
The effects of IL-6 on the expression and function of P-gp were investigated on two HepG2 and HuH7 cell lines possessing morphological characteristics of liver parenchymal cells (36). Contradictory results were obtained with a significant down-regulation of P-gp mediated efflux of rhodamine by IL-6 at 24 h and a corresponding decrease in MDR1 mRNA and immunoreactive P-gp expression in HuH7 cells, whereas no effect of IL-6 was observed in HepG2 cells.
Cultured rat hepatocytes were treated with 5 ng/mL of recombinant IL-6 for 0 to 24 h and analysed for the expression of mdr1 mRNA and its transcription rates (31). After 24 h, but not after 12 h, IL-6-treated hepatocytes displayed reduced levels of mdr1a and mdr1b by 35% and 65%, respectively, as compared to controls. In the mean time, transcription rates in IL-6-treated cells were suppressed by 22% and 26%, for mdr1a and mdr1b, respectively.
In hepatocytes isolated from rat livers, a 24h-treatment with IL-6 (1-10 ng/mL) resulted in a 20-38% reduction in the immunoreactive protein P-gp level, with a maximal suppression occurring at 5 ng/mL. At this IL-6 concentration, mdr1b mRNA was also significantly reduced. A downward trend was observed for mdr1a. The transport function of P-gp, evaluated by the verapamil-inhibitable efflux of rhodamine 123, was significantly reduced in IL-6-treated cells (37).
The ip treatment of mice with IL-6 alone (1,000-20,000 IU) resulted in a dose- and time-dependent significant decrease in the level of the P-gp (33). Regarding the corresponding genes, mdr1b was significantly decreased for a dose of 10,000 IU but not at a dose of 1,000 IU. A non-significant decrease was observed for mdr1a.
Effects of IL-1b alone on hepatic P-gp
The treatment of hepatocytes isolated from rat livers with IL-1b resulted in a decrease in both the expression and the function of P-gp (37). After a 3-day treatment with IL-1b (1-25 ng/mL), a reduction (32-75%) in the protein expression, which was maximal at 5 ng/mL was observed, whereas the mdr1a and mdr1b mRNAs were not modified. This IL-1b treatment depressed the transport function of P-gp, which was evidenced by a 34% increase in rhodamine accumulation.
In mice treated ip with isolated IL-1b (1,000-25,000 IU), IL-1b did not modify the levels of P-gp assayed by using the C219 antibody (33). Regarding the corresponding genes, mdr1a was significantly decreased, whereas mdr1b tended to be induced.
Effects of IFNγ alone on hepatic P-gp
Rat hepatocytes were treated with 1000 IU/mL of IFNγ or IFNγ for 3 days. Functionality was evaluated by using rhodamine transport and P-gp expression was studied by western blot using C219 antibody (38). The treatment of hepatocytes with IFNγ resulted in a decrease in P-gp functionality as seen by a significant increase in rhodamine incorporation, whereas western blot detection revealed an increase in P-gp expression. These results suggest that the decrease in P-gp function was not related to a reduced P-gp expression but may be due to a direct dysfunction by putative regulatory cofactors or to the impairment of the functional establishment of P-gp on the cell surface. On the contrary, IFNβ had no effect on P-gp expression and functionality.
In summary, studies investigating the effect of either LPS or turpentine injection as inflammation models in animals on the expression and/or functionality of P-gp in the liver showed that after induction of inflammation, mdr1a mRNA was constantly depressed (28, 29, 31-33), except in one study where it was not modified (30) (Table 1). Regarding mdr1b, contradictory results were observed with either an induction (28-30) or a decrease in mRNA expression (31, 32). The expression of the immunoreactive protein, when investigated, was most often decreased (30, 32, 33) or unchanged (34). When studied, the functionality was also depressed (28, 32).
Table 1: Influence of pro-inflammatory cytokines on the mdr gene, the P-glycoprotein and its functionality in the liver.

ND : not determined
In the studies investigating the effects of separated cytokines at the hepatic level, quite consensual results were obtained regarding the effect of IL-6 : decrease in the MDR1 (36) or mdr1b mRNAs (31, 33, 37), decrease in the level of the immunoreactive protein (36, 37) and depressed functionality (36, 37). Regarding TNFa, results were quite controversial, since the expression of the mdr mRNA and of the P-gp protein was increased (35), decreased (29) or unchanged (33). Only isolated results were obtained with IL-1b and IFNγ, which require confirmation. However, the results obtained with isolated cytokines should be considered cautiously, as a given cytokine modulates not only its own secretion but also the secretion of other cytokines.
Influence of cytokines on cerebral P-gp
Human brain endothelial BB19 cells were cultured with or without 1,000 IU/mL of rhTNFa (recombinant human TNFa ). The TNFa treatment did not significantly influence the mdr expression and resulted in a moderate decrease in the P-gp functionality (39).
Immortalized rat brain capillary endothelial GPNT cells were treated by various concentrations of TNFa for 4 days and the expression of mdr1 mRNA and protein was investigated together with the functionality (40). mdr1 mRNA and the protein did not response similarly to the TNFa treatment. An increase in mdr1a and mdr1b mRNAs was observed whereas TNFa had no influence on the expression of the protein. The treatment of cells with TNFa resulted in an increase in vinblastine accumulation into the cells. By pre-treating the cells with the P-gp inhibitor, GF120918, the effect of TNFa on the accumulation of vinblastine disappeared, whatever the TNFa concentration and the duration of contact, highly suggesting the direct involvement of TNFa in this process.
Mice were injected in the tail vein with Shi-like toxin type II (SLT-II) produced by an E. coli strain and the influence on P-gp expression and functionality were investigated together with the involvement of TNFa in this process (41). As reported previously (42), SLT-II was susceptible to induce the expression of cytokines, especially TNFa and IL-1 b, these two cytokines being able to play a role in SLT-II-induced histopathological lesions. By injecting SLT-II to mice, the expression of P-gp was increased in brain homogenates by a 2-fold factor 24 h after injection (41). The cerebral transport of doxorubicin was also investigated in mice treated with SLT-II, pre-treated or not with pentoxifylline. In case of pre-treatment with pentoxifylline, doxorubicin cerebral transport was increased by SLT-II then returned to control levels, suggesting that TNFa is involved in the modulation process of SLT-II.
The transfer of the human TNFa gene into human U373MG glioblastoma cells was used to investigate the effect of TNFα on the expression and functionality of P-gp (44). The TNFa -transfected cell-clone showed a marked reduction in P-gp expression when using the JSB-1 antibody. This reduction was dependent upon the amount of cytokines produced by the cells. In the TNF α -transfected clones, the uptake of rhodamine was increased as compared to the control and correlated to the level of P-gp expression. This decrease in functionality was also dependent on the amount of secreted cytokines into the clone.
After intracerebroventricular administration of E. coli- LPS in rats, P-gp function and expression were evaluated in the brain (28). Icv injection of LPS resulted in CNS inflammation. In LPS-treated rats, brain mdr1a mRNA levels were 50 % down-regulated at 6 h as compared to saline-treated rats and were back to the normal level by 24 h and 48 h, respectively. In LPS-treated rats, brain radioactivity and the plasma level of parent digoxin were increased, 24 h after injection of radiolabel led digoxin, suggesting a loss in cerebral P-gp function.
P-gp has been identified mainly in endothelial cells, but also on astrocytic endfeet (12) and in microglia (13). The influence of a wide variety of molecules known to induce astroglial activation was investigated in enriched astroglial cell cultures (43). The addition of recombinant mouse IL-6 to astroglial cells (0-40 ng/mL) resulted in a significant (68.8%) increase in P-gp intracellular content, but only at a high concentration (40 ng/mL). IL-1b or TNFa addition did not significantly alter this P-gp content.
In summary, studies in the brain are mainly limited to the effects of TNFa on P-gp (Table 2).
Table 2: Influence of pro-inflammatory cytokines on the mdr gene, the P-glycoprotein and its functionality in the brain.

ND : not determined
Results are controversial regarding the effects of cytokines on the expression of the mRNAs and of the protein, since the expression of mRNAs was increased (40), unchanged (39) or decreased (28) and the expression of the protein was increased (40, 41) decreased (44) or unchanged (40). On the contrary, regarding the functionality of P-gp, studies appear more unanimous and report a depressed functionality related to TNFa treatment (39-41, 44) or to LPS-induced inflammation model (28).
Influence of cytokines on intestinal P-gp
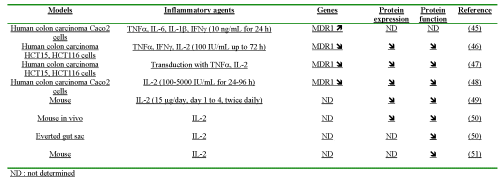
Human colon carcinoma Caco2 cells were harvested with or without addition of cytokines (TNFa, IL-1, IL-6, IFNγ) (45). After treatment of Caco2 cells for 24 h with 10 ng/mL of each cytokine, a 13-24% increase in MDR1 mRNA level was observed with IL-6, IFNγ or the combination of IL-6 and IFNγ. No change in the mRNA level was observed after the addition either of TNFa or IL-1b.
The influence of 100 IU/mL of cytokines (IFNγ, TNFa or IL-2), on the transducted human colon carcinoma cell lines HCT15 and HCT116, was investigated for MDR1 gene and P-gp expression (46). In both cell lines, MDR1 mRNA levels were decreased by the three cytokines in a time-dependent manner, the most striking effect being observed with TNFa. At 72 h after cytokine treatment, MDR1 levels were back to control levels. The expression of the immunoreactive P-gp was also depressed, which was in agreement with the reduction observed in the transcripts. Treatment with each cytokine resulted in an enhanced accumulation of doxorubicin, a P-gp substrate, suggesting a reduction in P-gp functionality.
Genes coding for human TNFa and IL-2 were introduced into the human colon carcinoma cell lines, HCT15 and HCT116, using the murine leukaemia virus (MLV)-based expression vectors constitutively expressing either TNFa or IL-2 under the control of the cytomegalovirus promoter (47). Then, the expression and the functionality of P-gp were compared to those in parental nontransduced cells or empty vector-harbouring clones. mdr1 mRNA level was reduced in TNFa− or IL-2 gene-transduced clones and the extent of reduction was associated with the level of either TNFa or IL-2. Similarly, transduction with TNFa or IL-2 resulted in a reduced P-gp expression, which ranged from 1.6- to 4.3-fold and 1.3- to 1.9-fold, in TNF-producing and in IL-2-producing HCT15 clones, respectively. Regarding the functionality, TNFa- or IL-2-transduction enhanced doxorubicin intracellular accumulation by 1.8- to 2.7-fold and 1.3- to 1.8-fold in HCT115 and HCT16 cells, respectively.
The effect of IL-2 on intestinal P-gp was investigated using various models. In vitro, in human colon carcinoma Caco2 cells incubated with IL-2 (100-1000-5000 IU/mL for 24 to 96 hours), P-gp function was decreased in relation with the time of contact and with the concentration of IL-2. In parallel, MDR1 mRNA level was significantly down-regulated by IL-2 (48). In vivo, a chronic treatment with IL-2 at a dose of 15 g twice daily for 4 days (49) or at a dose of 9 MIU/kg by i.p. injection twice daily during 4 days (50) resulted in a significant decrease in P-gp protein expression in the intestine. The effect of IL-2 was also evaluated on P-gp activity, either by comparing digoxin pharmacokinetics with or without IL-2 pre-treatment (49, 51), or by using mouse everted gut sacs with rhodamine 123 as P-gp substrate (50). In both cases, the pre-treatment with IL-2 resulted in a significant decrease in P-gp functionality.
In summary, all studies, except one (45) reporting a slight increase in MDR1 mRNA in Caco2 treated with IL-6 or IFNγ but not with TNFa or IL-1γ, describe a systematic decrease in mdr mRNA in the intestine with TNFa, IL-2 or IFNγ (46-48), a depressed expression of the immunoreactive protein with TNFa, IL-2 or IFN γ (46-51) and a depressed functionality (46-51) (Table 3).
Table 3: Influence of pro-inflammatory cytokines on the mdr gene, the P-glycoprotein and its functionality in the intestine.
ND : not determined
Mechanisms of modulation of P-glycoprotein gene or protein by cytokines at the molecular level
Regarding the mechanisms of modulation of P-gp by cytokines, little is known. Many studies have evaluated the effects of cytokines on the expression and activity of Pgp but very few have investigated the mechanisms involved at the molecular level.
The expression and functionality of Pgp can be controlled pre- or post-transcriptionally. Different mechanisms of control at variable steps may exist for Pgp such as modification of protein stability, mRNA stability, gene transcription and gene amplification. Among these, control at the mRNA level is the most frequently described (4). These different mechanisms may also intervene simultaneously.
The immune system is regulated by a very complicated network through cytokines and their receptors. Transcriptional activation of inflammatory response-genes, such as the genes for cytokines and their receptors and for acute phase proteins, are controlled by specific transcription factors binding the gene enhancers and promoters (52). Transcription factors such as NF-κB, NF-IL6, CREB/ATF, Jun-Fos, STAT and NF-AT are involved in the control of mRNA expression of genes involved in the acute phase response(52).
Several studies have investigated the involvement of these transcription factors in the modulation of mdr mRNA. It has been shown that the induction of mdr1 mRNA expression by IL6 in breast cancer cells correlates with the activation of C/EBPb (also called NF-IL6) and C/EBPδ, two members of the C/EBP family of transcription factors (53). Chen et al have investigated the implication of NF-IL6 in MDR1 gene activation using transfection of human breast cancer cells by C/EBPb or a dominant-negative form of this nuclear factor (54). They observed a C/EBPb interaction on the MDR1 promoter via the region within -128 and -75. Using deletions and mutations, they also demonstrated that the AP-1 box (-123 to -111) negatively regulated MDR1 activation by NF-IL6 and that the inverted CCAAT box (also called Y box, -82 to -73) was required for MDR1 activation by NF-IL6. Again, Combates et al have demonstrated that NF-IL6 might be an important candidate for mediating the induction of MDR1 gene in response to a variety of stimuli (55). For this, they co-transfected HepG2 cells with an NF-IL6 expression vector and a MDR1 promoter-driven CAT reporter construct and observed that the potential of NF-IL6 to stimulate the reporter gene activity was reduced after mutation or deletion of NF-IL6 recognition sequence in MDR1 promoter. In rat hepatocytes and hepatoma cells, Ros et al have investigated the mechanism of mdr1b gene up-regulation by TNFa, knowing that TNF α can signal through various pathways, such as NF-kB and p53, transcription factors for which binding sites in the mdr1b promoter have been identified. They demonstrated that NF-kB but not p53 was involved in the activation of mdr1b in both types of cells (56).
Using HCT15 colon cancer cells, Bentires-Alj et al have demonstrated that NF-kB was involved in the regulation of the mdr1 mRNA expression in drug resistance (57). They observed that NF-kB or P-glycoprotein inhibition led to increased apoptotic cell death in response to daunomycin treatment. Moreover, NF-kB inhibition increased daunomycin cell uptake and reduced mdr1 mRNA and P-glycoprotein expression in HCT15 cells. In P-gp overexpressing osteosarcoma cells, type I interferons, but not IFNγ, inhibited cell growth with a higher effect in MDR1 cells compared to parental cells. Moreover, the higher sensitivity of P-gp overexpressing cells to type I IFNs correlated with a higher expression of the activator of the transcription STAT2 and STAT3, two intracellular mediators of the type I IFN signalling pathways (58).
Some data in the literature may suggest that nuclear receptors could also be involved in the mechanism of P-gp modulation by cytokines. Nuclear receptors are a large family of proteins involved in key metabolic processes. Among these nuclear factors, PXR (pregnane X receptor, in rodents), also known as steroid xenobiotics receptor (SXR) in humans is able to regulate CYP3A transcription (59) in mice (60), in rabbit (61), in rat (62) and in humans (63) and is activated by most of the CYP3A inducers (64). It has also been shown that PXR was involved in the transcriptional regulation of multidrug resistance protein (MDR1), which encodes for the drug transporter P-glycoprotein. Synold et al demonstrated that the chemotherapeutic agent paclitaxel was able to activate SXR and enhanced P-gp mediated drug clearance (65). They showed in primary hepatocyte and colon cultures that several SXR agonists enhanced MDR1 expression, suggesting that MDR1 was a PXR target gene in liver and intestine. Using human colon carcinoma cells and the induction of Pgp by rifampicin, Geick et al investigated the 5'-upstream region of human MDR1 for the presence of potential PXR response elements (66). They identified a distinct PXR binding site, a DR4 nuclear receptor response element, essential for MDR1 induction by rifampicin. More recently, it was demonstrated that PXR was also expressed in the blood brain barrier and that dosing rats with pregnenolone-16alpha-carbonitrile (PCN) and dexamethasone increased P-gp expression in brain capillaries and up-regulated specific transport in capillaries (67). Regarding nuclear receptors, an interesting study using human primary hepatocyte culture, has shown that IL-6 was able to decrease the expression of PXR mRNA. IL-6 also decreased the rifampicin- and phenobarbital- mediated induction of CYP2B6, CYP2C8, CYP2C9 and CYP3A4 (68). Relation between cytokine, nuclear receptors and P-gp remains unclear. However, as CYP3A and MDR1 are often co-induced, one can imagine that cytokine and inflammation conditions modulate the expression of mdr mRNA via the modulation of the expression of nuclear receptors.
Conclusion
In the models of inflammation, the results may depend on the inflammation inducer used (LPS or turpentine). It has been shown that LPS injection preferentially induces IL-1b, IL-2, IL-6, TNFa and IFNγ release, whereas turpentine induces the release of IL-1b and IL-6. For this reason, the data on P-gp expression and/or function may differ according to the compound used to induce inflammation. Compared to models of inflammation, the treatment with a single cytokine appears to be a more precise approach to investigate the influence of a cytokine separately. However, injection of a separate cytokine has also its limitations. Indeed, cytokines are known to be involved not only in their own regulation but also in the regulation of other cytokines. For these reasons, the addition of high concentrations of a given cytokine in a cell culture medium or its injection to an animal induces a cascade of reactions that may alter the release of other cytokines.
The results also depend on in vivo or in vitro models used. This is obvious at the BBB level where mdr1b is not detectable in vivo, whereas it is expressed in cell cultures and can be modulated by TNFα. It is probably the case for other organs.
From the data in the literature (Tables 1, 2 and 3), it appears delicate to conclude definitely regarding the effect of cytokines on the expression and functionality of P-glycoprotein. However, despite of contradictory results, there is a general trend towards a decrease in both the expression of P-gp (mRNA and protein) and its functionality after treatment of cells or animals with pro-inflammatory cytokines. This trend is independent of the organ or anatomic barrier considered, at the hepatic, cerebral or intestinal level.
Regarding the mechanisms of modulation of P-gp by cytokines, some interesting studies indicate that transcription factors and nuclear receptors are probably involved in the regulation of expression and/or functionality of efflux proteins by cytokines. However, little is known on how cytokines modulate the expression of the MDR genes at the molecular level.
In total, it appears obvious that cytokines may interfere with P-gp. Hence, in pathological conditions, such as inflammation or infection where cytokines are released and involved, the expression and functionality of efflux proteins, like P-glycoprotein but also others like multi-drug associated protein (MRPs) and breast cancer resistance protein (BCRP), may be modulated with consequences for drug disposition and, consequently treatment efficacy. However, most of the studies investigating the influence of cytokines on P-gp have been conducted on in vitro models or in animals and the clinical significance of P-gp modulation in inflammation or infection is not known. Moreover, the cytokines released in such pathological conditions may also influence other pharmacokinetic factors, such as metabolism (CYP,...), with additional consequences on drug disposition.
References
Juliano, R. L., and Ling, V. A surface glycoprotein modulating drug permeability in Chinese hamster ovary cell mutants. Biochim Biophys Acta, 455: 152-62, 1976.
Thiebaut, F., Tsuruo, T., Hamada, H., Gottesman, M. M., Pastan, I., and Willingham, M. C. Cellular localization of the multidrug resistance gene product in normal human tissues. Proc Natl Acad Sci U S A, 84: 7735-8, 1987.
Dantzig, A. H., de Alwis, D. P., and Burgess, M. Considerations in the design and development of transport inhibitors as adjuncts to drug therapy. Adv Drug Deliv Rev, 55: 133-50, 2003.
Sukhai, M., and Piquette-Miller, M. Regulation of the multidrug resistance genes by stress signals. J Pharm Pharm Sci, 3: 268-80, 2000.
Greiner, B., Eichelbaum, M., Fritz, P., Kreichgauer, H. P., von Richter, O., Zundler, J., and Kroemer, H. K. The role of intestinal P-glycoprotein in the interaction of digoxin and rifampin. J Clin Invest, 104: 147-53, 1999.
Wadler, S., and Schwartz, E. L. Antineoplastic activity of the combination of interferonand cytotoxic agents against experimental and human malignancies: a review. Cancer Res., 50: 3473-86, 1990.
McRae, M. P., Brouwer, K. L. R., and Kashuba, A. D. M. Cytokine regulation of P-glycoprotein. Drug Metab Rev, 35: 19-33, 2003.
Smit, J. J., Schinkel, A. H., Oude-Elferink, R. P., Groen, A. K., Wagenaar, E., van Deemter, L., Mol, C. A., Ottenhoff, R., van der Lugt, N. M., van Roon, M. A., Offerhaus, G. J. A., Berns, A. J. M., and Borst, P. Homozygous disruption of the murine mdr2 P-glycoprotein gene leads to a complete absence of phospholipid from bile and to liver disease. Cell, 75: 451-62, 1993.
Schinkel, A. H., Wagenaar, E., van Deemter, L., Mol, C. A., and Borst, P. Absence of the mdr1a P-Glycoprotein in mice affects tissue distribution and pharmacokinetics of dexamethasone, digoxin, and cyclosporin A. J Clin Invest, 96: 1698-705, 1995.
Barrand, M. A., Robertson, K. J., and von Weikersthal, S. F. Comparisons of P-glycoprotein expression in isolated rat brain microvessels and in primary cultures of endothelial cells derived from microvasculature of rat brain, epididymal fat pad and from aorta. FEBS Lett, 374: 179-83, 1995.
Lum, B. L., and Gosland, M. P. MDR expression in normal tissues. Pharmacologic implications for the clinical use of P-glycoprotein inhibitors. Hematol Oncol Clin North Am, 9: 319-36, 1995.
Golden, P. L., and Pardridge, W. M. Brain microvascular P-glycoprotein and a revised model of multidrug resistance in brain. Cell Mol Neurobiol, 20: 165-81, 2000.
Lee, G., Schlichter, L., Bendayan, M., and Bendayan, R. Functional expression of P-glycoprotein in rat brain microglia. J Pharmacol Exp Ther, 299: 204-12, 2001.
Rao, V. V., Dahlheimer, J. L., Bardgett, M. E., Snyder, A. Z., Finch, R. A., Sartorelli, A. C., and Piwnica-Worms, D. Choroid plexus epithelial expression of MDR1 P glycoprotein and multidrug resistance-associated protein contribute to the blood-cerebrospinal-fluid drug-permeability barrier. Proc Natl Acad Sci U S A, 96: 3900-5, 1999.
Lin, J. H., and Yamazaki, M. Role of P-glycoprotein in pharmacokinetics: clinical implications. Clin Pharmacokinet, 42: 59-98, 2003.
van Asperen, J., van Tellingen, O., and Beijnen, J. H. The role of mdr1a P-glycoprotein in the biliary and intestinal secretion of doxorubicin and vinblastine in mice. Drug Metab Dispos., 28: 264-7, 2000.
Schinkel, A. H., Wagenaar, E., Mol, C. A., and van Deemter, L. P-glycoprotein in the blood-brain barrier of mice influences the brain penetration and pharmacological activity of many drugs. J Clin Invest, 97: 2517-24, 1996.
Mayer, U., Wagenaar, E., Beijnen, J. H., Smit, J. W., Meijer, D. K., van Asperen, J., Borst, P., and Schinkel, A. H. Substantial excretion of digoxin via the intestinal mucosa and prevention of long-term digoxin accumulation in the brain by the mdr 1a P-glycoprotein. Br J Pharmacol, 119: 1038-44, 1996.
Schinkel, A. H., Smit, J. J., van Tellingen, O., Beijnen, J. H., Wagenaar, E., van Deemter, L., Mol, C. A., van der Valk, M. A., Robanus-Maandag, E. C., and te Riele, H. P. Disruption of the mouse mdr1a P-glycoprotein gene leads to a deficiency in the blood-brain barrier and to increased sensitivity to drugs. Cell, 77: 491-502, 1994.
Gottesman, M. M., and Pastan, I. Biochemistry of multidrug resistance mediated by the multidrug transporter. Annu Rev Biochem, 62: 385-427, 1993.
Munoz-Fernandez, M. A., and Fresno, M. The role of tumour necrosis factor, interleukin 6, interferon-gamma and inducible nitric oxide synthase in the development and pathology of the nervous system. Progress Neurobiol, 56: 307-40, 1998.
Abdel-Razzak, Z., Loyer, P., Fautrel, A., Gautier, J. C., Corcos, L., Turlin, B., Beaune, P., and Guillouzo, A. Cytokines down-regulate expression of major cytochrome P-450 enzymes in adult human hepatocytes in primary culture. Mol Pharmacol, 44: 707-15, 1993.
Parmentier, J. H., Schohn, H., Bronner, M., Ferrari, L., Batt, A. M., Daucat, M., and Kremers, P. Regulation of CYP4A1 and peroxisome proliferator-activated receptor alpha expression by interleukin-1beta, interleukin-6 and dexamethasone in cultured fetal rat hepatocytes. Biochem Pharmacol, 54: 889-98, 1997.
Turrin, N. P., Gayle, D., Ilyin, S. E., Flynn, M. C., Langhans, W., Schwartz, G. J., and Plata-Salaman, C. R. Pro-inflammatory and anti-inflammatory cytokine mRNA induction in the periphery and brain following intraperitoneal administration of bacterial lipopolysaccharide. Brain Res Bull, 54: 443-53, 2001.
De Simoni, M. G., Terreni, L., Chiesa, R., Mangiarotti, F., and Forloni, G. L. Interferon-gamma potentiates interleukin (IL)-6 and tumor necrosis factor-alpha but not IL-1beta induced by endotoxin in the brain. Endocrinology, 138: 5220-6, 1997.
Teeter, L. D., Estes, M., Chan, J. Y., Atassi, H., Sell, S., Becker, F. F., and Kuo, M. T. Activation of distinct multidrug-resistance (P-glycoprotein) genes during rat liver regeneration and hepatocarcinogenesis. Mol Carcinog, 8: 67-73, 1993.
Hirsch-Ernst, K. I., Ziemann, C., Foth, H., Kozian, D., Schmitz-Salue, C., and Kahl, G. F. Modulation of P-glycoprotein and mdr1b mRNA expression by growth factors in primary rat hepatocyte culture. Biochem Biophys Res Commun, 215: 179-85, 1995.
Goralski, K. B., Hartmann, G., Piquette-Miller, M., and Renton, K. W. Downregulation of mdr1a expression in the brain and liver during CNS inflammation alters the in vivo disposition of digoxin. Br J Pharmacol, 139: 35-48, 2003.
Ando, H., Nishio, Y., Ito, K., Nakao, A., Wang, L., Zhao, Y. L., Kitaichi, K., Takagi, K., and Hasegawa, T. Effect of endotoxin on P-glycoprotein-mediated biliary and renal excretion of rhodamine-123 in rats. Antimicrob Agents Chemother, 45: 3462-7, 2001.
Vos, T. A., Hooiveld, G. J., Koning, H., Childs, S., Meijer, D. K., Moshage, H., Jansen, P. L., and Muller, M. Up-regulation of the multidrug resistance genes, Mrp1 and Mdr1b, and down-regulation of the organic anion transporter, Mrp2, and the bile salt transporter, Spgp, in endotoxemic rat liver. Hepatology, 28: 1637-44, 1998.
Sukhai, M., Yong, A., Kalitsky, J., and Piquette-Miller, M. Inflammation and interleukin-6 mediate reductions in the hepatic expression and transcription of the mdr1a and mdr1b Genes. Mol Cell Biol Res Commun, 4: 248-56, 2000.
Piquette-Miller, M., Pak, A., Kim, H., Anari, R., and Shahzamani, A. Decreased expression and activity of P-glycoprotein in rat liver during acute inflammation. Pharm Res, 15: 706-11, 1998.
Hartmann, G., Kim, H., and Piquette-Miller, M. Regulation of the hepatic multidrug resistance gene expression by endotoxin and inflammatory cytokines in mice. Int Immunopharmacol, 1: 189-99, 2001.
Trauner, M., Arrese, M., Soroka, C. J., Ananthanarayanan, M., Koeppel, T. A., Schlosser, S. F., Suchy, F. J., Keppler, D., and Boyer, J. L. The rat canalicular conjugate export pump (Mrp2) is down-regulated in intrahepatic and obstructive cholestasis. Gastroenterology, 113: 255-64, 1997.
Hirsch-Ernst, K. I., Ziemann, C., Foth, H., Kozian, D., Schmitz-Salue, C., and Kahl, G. F. Induction of mdr1b mRNA and P-glycoprotein expression by tumor necrosis factor alpha in primary rat hepatocyte cultures. J Cell Physiol, 176: 506-15, 1998.
Lee, G., and Piquette-Miller, M. Influence of IL-6 on MDR and MRP-mediated multidrug resistance in human hepatoma cells. Can J Physiol Pharmacol, 79: 876-84, 2001.
Sukhai, M., Yong, A., Pak, A., and Piquette-Miller, M. Decreased expression of P-glycoprotein in interleukin-1beta and interleukin-6 treated rat hepatocytes. Inflamm Res, 50: 362-70, 2001.
Akazawa, Y., Kawaguchi, H., Funahashi, M., Watanabe, Y., Yamaoka, K., Hashida, M., and Takakura, Y. Effect of interferons on P-glycoprotein-mediated rhodamine-123 efflux in cultured rat hepatocytes. J Pharm Sci, 91: 2110-5, 2002.
Mandi, Y., Ocsovszki, I., Szabo, D., Nagy, Z., Nelson, J., and Molnar, J. Nitric oxide production and MDR expression by human brain endothelial cells. Anticancer Res, 18: 3049-52, 1998.
Theron, D., Barraud de Lagerie, S., Tardivel, S., Pelerin, H., Demeuse, P., Mercier, C., Mabondzo, A., Farinotti, R., Lacour, B., Roux, F., and Gimenez, F. Influence of tumor necrosis factor-alpha on the expression and function of P-glycoprotein in an immortalised rat brain capillary endothelial cell line, GPNT. Biochem Pharmacol, 66: 579-87, 2003.
Zhao, Y. L., Du, J., Kanazawa, H., Cen, X. B., Takagi, K., Kitaichi, K., Tatsumi, Y., Takagi, K., Ohta, M., and Hasegawa, T. Shiga-like toxin II modifies brain distribution of a P-glycoprotein substrate, doxorubicin, and P-glycoprotein expression in mice. Brain Res, 956: 246-53, 2002.
Ramegowda, B., Samuel, J. E., and Tesh, V. L. Interaction of Shiga toxins with human brain microvascular endothelial cells: cytokines as sensitizing agents. J Infect Dis, 180: 1205-13, 1999.
Monville, C., Fages, C., Feyens, A. M., D'Hondt, V., Guillet, C., Vernallis, A., Gascan, H., and Peschanski, M. Astroglial expression of the P-glycoprotein is controlled by intracellular CNTF. BMC Cell Biol, 3: 20, 2002.
Walther, W., Stein, U., and Pfeil, D. Gene transfer of human TNF alpha into glioblastoma cells permits modulation of mdr1 expression and potentiation of chemosensitivity. Int J Cancer, 61: 832-9, 1995.
Bertilsson, P. M., Olsson, P., and Magnusson, K. E. Cytokines influence mRNA expression of cytochrome P450 3A4 and MDRI in intestinal cells. J Pharm Sci, 90: 638-46, 2001.
Stein, U., Walther, W., and Shoemaker, R. H. Modulation of mdr1 expression by cytokines in human colon carcinoma cells: an approach for reversal of multidrug resistance. Br J Cancer, 74: 1384-91, 1996.
Stein, U., Walther, W., and Shoemaker, R. H. Reversal of multidrug resistance by transduction of cytokine genes into human colon carcinoma cells. J Natl Cancer Inst, 88: 1383-92, 1996.
Belliard, A. M., Tardivel, S., Farinotti, R., Lacour, B., and Leroy, C. Effect of hr-IL2 treatment on intestinal P-glycoprotein expression and activity in Caco-2 cells. J Pharm Pharmacol, 54: 1103-9, 2002.
Bonhomme-Faivre, L., Pelloquin, A., Tardivel, S., Urien, S., Mathieu, M. C., Castagne, V., Lacour, B., and Farinotti, R. Recombinant interleukin-2 treatment decreases P-glycoprotein activity and paclitaxel metabolism in mice. Anticancer Drugs, 13: 51-7, 2002.
Veau, C., Faivre, L., Tardivel, S., Soursac, M., Banide, H., Lacour, B., and Farinotti, R. Effect of interleukin-2 on intestinal P-glycoprotein expression and functionality in mice. J Pharmacol Exp Ther, 302: 742-50, 2002.
Castagne, V., Bonhomme-Faivre, L., Urien, S., Reguiga, M. B., Soursac, M., Gimenez, F., and Farinotti, R. Effect of recombinant interleukin-2 pretreatment on oral and intravenous digoxin pharmacokinetics and P-glycoprotein activity in mice. Drug Metab Dispos., 32: 168-71, 2004.
Akira, S. IL-6-regulated transcription factors. Int J Biochem Cell Biol, 29: 1401-18, 1997.
Conze, D., Weiss, L., Regen, P. S., Bhushan, A., Weaver, D., Johnson, P., and Rincon, M. Autocrine production of interleukin 6 causes multidrug resistance in breast cancer cells. Cancer Res, 61: 8851-8, 2001.
Chen, G. K., Sale, S., Tan, T., Ermoian, R. P., and Sikic, B. I. CCAAT/enhancer-binding protein beta (nuclear factor for interleukin 6) transactivates the human MDR1 gene by interaction with an inverted CCAAT box in human cancer cells. Mol Pharmacol, 65: 906-16, 2004.
Combates, N. J., Rzepka, R. W., Chen, Y. N., and Cohen, D. NF-IL6, a member of the C/EBP family of transcription factors, binds and trans-activates the human MDR1 gene promoter. J Biol Chem, 269: 29715-9, 1994.
Ros, J. E., Schuetz, J. D., Geuken, M., Streetz, K., Moshage, H., Kuipers, F., Manns, M. P., Jansen, P. L., Trautwein, C., and Muller, M. Induction of Mdr1b expression by tumor necrosis factor-alpha in rat liver cells is independent of p53 but requires NF-kappaB signaling. Hepatology, 33: 1425-31, 2001.
Bentires-Alj, M., Barbu, V., Fillet, M., Chariot, A., Relic, B., Jacobs, N., Gielen, J., Merville, M. P., and Bours, V. NF-kappaB transcription factor induces drug resistance through MDR1 expression in cancer cells. Oncogene, 22: 90-7, 2003.
Manara, M. C., Serra, M., Benini, S., Picci, P., and Scotlandi, K. Effectiveness of Type I interferons in the treatment of multidrug resistant osteosarcoma cells. Int J Oncol, 24: 365-72, 2004.
Quattrochi, L. C., and Guzelian, P. S. Cyp3A regulation: from pharmacology to nuclear receptors. Drug Metab Dispos., 29: 615-22, 2001.
Kliewer, S. A., Moore, J. T., Wade, L., Staudinger, J. L., Watson, M. A., Jones, S. A., McKee, D. D., Oliver, B. B., Willson, T. M., Zetterstrom, R. H., Perlmann, T., and Lehmann, J. M. An orphan nuclear receptor activated by pregnanes defines a novel steroid signaling pathway. Cell, 92: 73-82, 1998.
Savas, U., Wester, M. R., Griffin, K. J., and Johnson, E. F. Rabbit pregnane X receptor is activated by rifampicin. Drug Metab Dispos., 28: 529-37, 2000.
Zhang, H., LeCulyse, E., Liu, L., Hu, M., Matoney, L., Zhu, W., and Yan, B. Rat pregnane X receptor: molecular cloning, tissue distribution, and xenobiotic regulation. Arch Biochem Biophys, 368: 14-22, 1999.
Bertilsson, G., Heidrich, J., Svensson, K., Asman, M., Jendeberg, L., Sydow-Backman, M., Ohlsson, R., Postlind, H., Blomquist, P., and Berkenstam, A. Identification of a human nuclear receptor defines a new signaling pathway for CYP3A induction. Proc Natl Acad Sci U S A, 95: 12208-13, 1998.
Ekins, S., and Erickson, J. A. A pharmacophore for human pregnane X receptor ligands. Drug Metab Dispos., 30: 96-9, 2002.
Synold, T. W., Dussault, I., and Forman, B. M. The orphan nuclear receptor SXR coordinately regulates drug metabolism and efflux. Nat Med, 7: 584-90, 2001.
Geick, A., Eichelbaum, M., and Burk, O. Nuclear receptor response elements mediate induction of intestinal MDR1 by rifampin. J Biol Chem, 276: 14581-7, 2001.
Bauer, B., Hartz, A. M., Fricker, G., and Miller, D. S. Pregnane X receptor up-regulation of P-glycoprotein expression and transport function at the blood-brain barrier. Mol Pharmacol, 66: 413-9, 2004.
Pascussi, J. M., Gerbal-Chaloin, S., Pichard-Garcia, L., Daujat, M., Fabre, J. M., Maurel, P., and Vilarem, M. J. Interleukin-6 negatively regulates the expression of pregnane X receptor and constitutively activated receptor in primary human hepatocytes. Biochem Biophys Res Commun, 274: 707-13, 2000.
Corresponding Author: Christine Fernandez, Faculté de Pharmacie, Pharmacie clinique, 5, rue Jean Baptiste Clément, 92296 Châtenay-Malabry, France. christine.fernandez@psl.ap-hop-paris.fr
Published by the Canadian Society for Pharmaceutical Sciences.
Copyright © 1998 by the Canadian Society for Pharmaceutical Sciences.
http://www.ualberta.ca/~csps