J Pharm Pharmaceut Sci (www.ualberta.ca/~csps) 7(3):353-358, 2004
Isoforms of serum γ-glutamyltransferase in epileptic patients treated with enzyme-inducing anticonvulsant drugs.
María Tutor-Crespo, Jesús Hermida, Carlos Tutor1
Laboratorio Central. Hospital Clínico Universitario. Santiago de Compostela. SpainReceived 17 June 2004, Revised 5 October 2004, Accepted 4 November 2004, Published 15 November 2004
PDF Version
Abstract
PURPOSE: The increase of serum activity of g-glutamyltranferase (gGT) through the action of enzyme-inducing anticonvulsant drugs has been widely documented; however, the behaviour of its multiple forms and its relationship with the degree of enzyme induction has received little coverage. This subject is the major aim of our paper. METHODS. An electrophoretic study of the serum gGT isoforms was made in 90 adult epileptic patients under chronic treatment with phenobarbital, phenytoin and carbamazepine in polytherapy. RESULTS. A significant correlation was found (p<0.001) between the drug score and urinary excretion of D-glucaric acid (DGA) (r=0.773), total gGT (r=0.382), gGT1 (r=0.398) and gGT2 (r=0.361). In a group of 11 patients with the gGT3 isoforms, considered a sensitive test for cholestasis, serum activities of total gGT, gGT1, gGT2 and 5´-nucleotidase were found that were significantly higher than in the 79 patients without the gGT3 isoform (p<0.001); however, for the drug score and excretion of DGA, no significant differences were found, suggesting a similar degree of enzyme induction in both groups of patients. CONCLUSIONS. The presence of cholestasis, at least biochemically detectable in some of these patients, appears to be a factor of paramount importance when interpreting the effect of enzyme-inducing anticonvulsant drugs on serum gGT. This fact may contribute towards explaining its highly varied response to the administration of these drugs.
Introduction
Enzyme induction by drugs mostly concerns the enzyme systems involved in drug metabolism. γ-Glutamyltransferase (γGT, EC 2.3.2.2) is a membrane-bound enzyme that participates in the metabolism of glutathione, cleaving its γ-glutamyl peptide bond and transferring the glutamyl moiety to acceptor molecules. As a result, and via glutathione, γGT is indirectly involved in drug metabolism (1).
Hepatic γGT is induced by phenobarbital-type enzyme-inducing agents, and increased serum enzyme activities have been described in patients treated with anticonvulsant drugs by different authors (2-4). However, as well as the induction of the enzyme protein synthesis in the liver, other mechanisms such as alterations in the lipid composition of plasma membranes may contribute to the increase of γGT serum activity in these patients (5,6). γGT has a well-documented enzyme heterogeneity (7), and its serum multiple forms have been previously studied in patients treated with anticonvulsant drugs (8-10), although this subject has been dealt with in a horizontal manner. The urinary excretion of D-glucaric acid (DGA), an end product of carbohydrate metabolism in humans produced via the glucuronic acid pathway has been widely used as an indirect enzyme induction marker and it is significantly increased by these drugs (1).
In our article, we present the results obtained for the isoforms of serum γGT in a group of adult epileptic patients treated with phenobarbital, phenytoin and carbamazepine, in an attempt to clarify its relationship with the degree of enzyme induction evaluated by means the drug score and the urinary excretion DGA.
Material and Methods
A group of 90 epileptic patients (56 males and 34 female) with a mean age (± SEM) of 38.0 ± 1.5 years was studied, who had been treated for more than 10 years with phenobarbital (n=60), phenytoin (n=70) and carbamazepine (n=33). In all cases, there was adequate therapeutic compliance, and no additional pharmacological treatment was received. As the anticonvulsant drugs were administered in polytherapy, the dose was expressed as units/day, according to a drug score in which one unit corresponded to every 30 mg of phenobarbital, 50 mg of phenytoin and 100 mg of carbamazepine (2, 11). After an overnight fast, venous blood and urine samples were taken before the morning administration of anticonvulsant drugs, whose doses had not been modified for at least three months beforehand. The control group comprised 49 medication-free clinically healthy individuals (30 male and 19 female) with a mean age of 36.7 ± 1.6 years. Pregnant women or those who were taking oral contraceptives were excluded from both the control and patients group.
The serum enzyme activities of γGT were determined using γ-glutamyl-3-carboxi-4-nitroanilide as substrate using commercial reagents from Roche Diagnostics. The residual enzymatic activity after treatment of the serum samples with butanol was determined according to a previously described procedure (12, 13). Electrophoretic separation of the γGT multiple forms was carried out on cellulose acetate plates (8). Serum activity of alcohol dehydrogenase (ADH, EC 1.1.1.1) was determined spectophotometrically (14), and serum a -glutathione-S-transferase (a GST, EC 2.5.1.18) was determined using an enzyme immunoassay commercialized by Biotrin International. The serum activities of aspartate aminotransferase (AST, EC 2.6.1.1) and alanine aminotransferase (ALT, EC 2.6.1.2) were determined according to the recommendations of the Spanish Clinical Biochemistry Society. The activity of 5´-nucleotidase (5´NU, EC 3.1.3.5) was determined using commercial reagents from Sigma Diagnostics. Urinary DGA was determined using an enzymatic procedure (15), and the results were expressed as the ratio of DGA to creatinine urinary concentrations (16). The variations of the different variables studied in the patient group were calculated using the expression: Variation (%) = 100 (median patients - median controls) / median controls.
Statistical analysis of the data was carried out using Microsoft Excel (v.5.0). The Kolmogorov-Smirnov test was applied to check for normality. Parametric tests were used when the data had a Gaussian distribution (Student's t test and Pearson's correlation coefficient); otherwise, non-parametric tests were used (Mann-Whitney test and Spearman's correlation coefficient). The results were expressed as mean ± SEM (median).
RESULTS
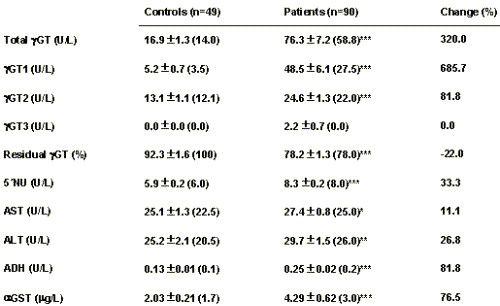
The urinary excretion of DGA in the patient group was 276.4±22.8 (244.5) mmol/g creatinine, and in the control group 18.5±1.0 (16.7) mmol/g creatinine, with a highly significant difference between both groups (p<0.001). In 93% of the patients, urinary DGA was higher than the upper reference limit. For serum γGT, the patient group presented a significantly higher activity than the control group (Table 1), with 81% of the cases presenting activities higher than the corresponding upper reference limit for their sex.
Table 1: Serum enzyme activities in the control and patient groups.
Significance: *p<0.05; **p<0.01; ***p<0.001
Using the electrophoretic technique applied, two isoforms of serum γGT were separated in the control subjects, one with a 1-globulin mobility (γGT1) and another with a 2-globulin mobility (γGT2) with higher relative activity (see Table 1). In the patient group, a significant increase (p<0.001) was found for total γGT, γGT1 and γGT2 activity, with a significant increase (p<0.001) in the relative proportion of γGT1 as compared to the control group (53.9±2.2% vs. 27.0±2.5%). In 11 of the patients studied (12.2%) an additional isoform was found with b-globulin mobility (γGT3) that is considered as a sensitive test for cholestasis (17, 18). Amongst the patients studied, the total γGT activities had a significant correlation with the activities of the γGT1 isoform (r=0.963, p<0.001) and γGT2 isoform (r=0.630, p<0.001), as well as with the relative proportion as a percentage of γGT1 (r=0.765, p<0.001). These results show that the increases in serum γGT activity in these patients is mainly due to the γGT1 isoform.
Table 1 shows the results obtained for γGT and its multiple forms, as well as for the other biochemical variables studied, in the control and patient groups. With the exception of the activity of the γGT3 isoform, significant differences were found between both groups for all of the variables, although their variations with relation to the control group are highly varied. The residual γGT activity after treatment with butanol was significantly higher in the control group (p<0.001), which suggests that the γGT1 isoform is affected more than γGT2. This was confirmed by electrophoretically fractioning the γGT isoforms in serum samples from patients before and after treatment with butanol. Moreover, in the patient group a significant negative correlation was found between the residual γGT activity and the relative proportion as a percentage of the γGT1 isoform (r=-0.559, p<0.001).
A highly significant correlation was found in the patient group between the urinary excretion of DGA and the drug score (r=0.773, p<0.001). Table 2 shows the correlation coefficients for γGT and its multiple forms with the drug score, urinary DGA and serum 5´NU activity.
Table 2: Relationship between serum γGT isoforms and other variables in the patient group.
Significance: *p<0.005; **p<0.001
Significant correlations were also found between γGT and AST, ALT, ADH and α GST (p<0.005).
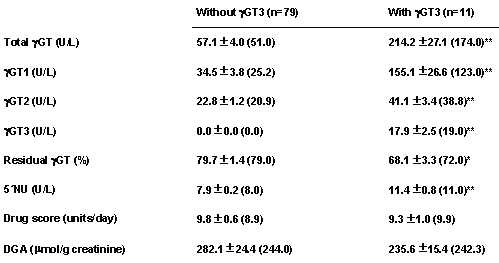
Table 3 shows the results obtained from making a comparison of the patients studied depending on whether or not they had the γGT3 isoform.
Table 3: Serum activities of γGT isoforms and other variables in the groups of patients without and with the γGT3 isoform.
Significance: *p<0.01; ** p<0.001
Although no significant differences were found for the drug score and the urinary excretion of DGA, the serum activities of total γGT, γGT1, γGT2 and 5´NU were significantly higher in the patients who presented the γGT3 isoform; however, the increase was much higher for γGT1 (388%) than for γGT2 (86%).A dichotomy of the patients according to sex not appear to offer any information with additional interest.
Discussion
Judging by the results obtained for the urinary excretion of DGA, the studied epileptic patients presented a high degree of enzyme induction. The degree of enzyme induction produced by anticonvulsant drugs is dose-dependent (19), and the excretion of DGA revealed a high correlation with the drug score used (r=0.765, p<0.001), which may reflect the enzyme-inducing capacity of the anticonvulsant drugs administered in polytherapy (11, 20).
Similarly, the patients studied presented a significant increase in serum γGT activity (see Table 1). Treatment with phenobarbital-type enzyme-inducing agents produced a strong induction of γGT in the hepatic plasma membranes (21); however, enzyme induction is not the only mechanism that may be involved in the increase of γGT serum activity (1). Another important factor is the possible effect of these drugs on the lipid composition of plasma membranes, by increasing the phospholipids/cholesterol molar ratio (5, 6). This ratio is the most determining factor for the fluidity of the membranes, as well as the interaction between different intramembrane composites, and as a result, the drug treatment may facilitate the solubilization and release of γGT hydrophobic forms from the plasma membranes to the extracellular milieu (5, 6). In blood, the hydrophobic forms of γGT may form aggregates with the lipoprotein X, and low (LDL) and very low density lipoproteins (VLDL), with an electrophoretic mobility similar to that of the β-γ globulins, whereas aggregates with high density lipoproteins (HDL) have α 1 mobility (22, 23). In clinically healthy individuals, the predominant γGT forms are hydrophylic (24) and do not form complexes with the lipoproteins (22, 25), although they may have a1 mobility (22).
The patients studied presented an increase in the relative proportion of the α 1 mobility isoform (γGT1), whose hydrophobic nature explains the lower residual enzymatic activity after treatment of serum samples with butanol as compared to the control group, in which enzymatic activity was practically unchanged (see Table 1). The anticonvulsant drugs may favour the solubilization of γGT bound to the plasma membranes and their release to the blood stream, where its hydrophobic nature allows them to form complexes with HDL constituting the γGT1. This isoform could correspond to the intermediate relative molecular fraction, and the γGT2 isoform to the low relative molecular form described by other authors (7, 9). Although the membrane-bound 5´NU is not induced by phenobarbital (26), its serum activity is also significantly increased in the patient group, having a better correlation with the γGT1 than with the γGT2 isoform (table 2). The serum activity of γGT1 increased greatly in the patients studied (686%), whereas the possibly hydrophilic form γGT2 had a much more moderate increase (82%), similar to that of the cytosolic enzymes ADH (82%) and α GST (77%) which are considered sensitive markers for hepatocellular damage (14, 27). The alteration of the lipid composition and the permeability of plasma membranes by the administered anticonvulsant drugs may also favour the release of cytosolic enzymes into the bloodstream.
The increases found in the group of patients for the cytosolic/mitochondrial AST (11%) and cytosolic ALT (27%) serum activities were lower than those obtained for ADH and αGST. This may be due to the preferable localization of both aminotransferases in azinar zone 1 (periportal), whereas the hepatic injury produced by drugs is mainly localized in azinar zone 3 (centrilobular), where the concentration of drugs and their metabolites is higher (28, 29). In turn, ADH is preferably localized in the centrilobular region (30), and αGST is equally distributed throughout the liver lobe (31). αGST, one of a family of detoxication enzymes, may be induced by phenobarbital although with a large organ and species variability (32).
The increase of serum 5´NU is specific for cholestatic liver injury, as the detergent action of bile acids on the canalicular membrane is the only mechanism for enzyme release into plasma (29). In the group of patients with the γGT3 isoform, which as previously mentioned is a sensitive test for cholestasis (17, 18), significant increases in 5´NU were found, as well as for total γGT, γGT1 and γGT2 (p<0.001), as compared to the patients who did not have γGT3 isoform (see Table 3). However, no significant differences were found for the drug score or urinary excretion of DGA, which suggests a similar degree of enzyme induction in both groups of patients. This γGT3 isoform could correspond to the high relative molecular mass fraction formed by aggregates of γGT with LDL, VLDL or membrane fragments (7, 9). The prevalence of 12% for the presence of γGT3 isoform in our group of patients is clearly lower than that of 21% detected by Kok et al. in a group of 38 psychiatric patients treated with phenobarbital and phenytoin (8). Possibly, the patients studied by these authors were concomitantly treated with neuroleptic, antipsychotic or antidepressant drugs.
Different factors have been indicated that should be taken into account when interpreting γGT serum activity as an index for enzyme induction in patients treated with anticonvulsant drugs (3). Our results indicate that the presence of cholestasis, at least biochemically detectable in some of these patients, is a factor of extreme importance in explaining the increase in serum γGT through the action of anticonvulsant drugs. In a similar way to that described for the ingestion of alcohol (33), the degree of response of γGT to the administration of these drugs appears to be highly variable, which would explain the discrete correlation found between its serum enzymatic activity and the drug score (see Table 2). Similarly, in a previous study we found that in patients treated with anticonvulsant drugs there was no significant correlation between the changes in the serum activity of γGT and those of drug score (20). As a result, the serum activity of total γGT is of no use as a marker for enzyme induction, and separating the multiple forms of the enzyme does not appear to offer any additional information of practical interest in this sense. However, in epileptic patients, the evaluation of the γGT3 isoform may be advisable in its biochemical monitoring as a sensitive marker of cholestasis produced by anticonvulsant drugs.
References
Batt AM, Siest G, Magdalou J, Galteau MM. Enzyme induction by drugs and toxins. Clin Chim Acta 1992;209:109-121.
Rosalki SB. Plasma enzyme changes and their interpretation in patients receiving anticonvulsant and enzyme-inducing drugs. In: Richens A, Woodford FP, editors. Anticonvulsant Drugs and Enzyme Induction. Associated Scientific Publishers, Amsterdam 1976. p. 27-35.
Braide SA, Davies TJ. Factors that affect the induction of gamma glutamyltransferase in epileptic patients receiving anticonvulsant drugs. Ann Clin Biochem 1987;24:391-399.
Aldenhövel HG. The influence of long-term anticonvulsant therapy with diphenylhydantoin and carbamazepine on serum gamma-glutamyltransferase, aspartate aminotransferase, alanine aminotransferase and alkaline phosphatase. Eur Arch Psychiatr Neurol Sci 1988;237:312-316.
Ratanasavanh D, Magdolou J, Antoine B, Galteau MM, Siest G. Gamma glutamyltransferase activity of liver plasma membranes in phenobarbital-treated rabits. Pharmacol Res Commun 1981;13:909-919.
Ratanasavanh D, Tazi A, Gaspart E, Jacquier A, Notter D, Galteau MM, Siest G. Hepatic gamma-glutamyltransferase release: Effect of bile salts and membrane structure modifications. In: Siest G, Eusghen C, editors. Gamma-glutamyltransferases. Advances in Biochemical Pharmacology. Masson, Paris 1982. p. 92-103.
Wenham PR, Horn DB, Smith AF. Physical properties of g-glutamyltransferase in human serum. Clin Chim Acta 1984;141:205-218.
Kok PJMJ, Seidel B, Holtkamp HC, Huisman J. A new procedure for the visualization of multiple forms of gamma-glutamyltransferase. Results in normals, patients receiving enzyme-inducing drugs and patients having liver parenchymal lesions. Clin Chim Acta 1978;90:209-216.
Wenham PR, Horn DB, Smith AF. Multiple forms of g-glutamyltransferase: A clinical study. Clin Chem 1985;31:569-573.
ellini M, Tumino E, Giordani R, Fabrini G, Costa F, Galli R, Rucco M, Belcari C, Michelassi C, Murri L, Maltinti G, Marchi S. Serum gamma-glutamyltransferase isoforms in alcoholic liver disease. Alcohol Alcohol 1997;32:259-266.
Richens A, Rowe DJF. Disturbance of calcium metabolism by anticonvulsant drugs. Br Med J 1970;4:73-76.
Beck PR. Butanol extraction of serum and urinary gamma-glutamyltransferase. In: Goldberg DM, Wilkinson SH, editors. Enzymes in Health and Disease. Karger, Basel 1978. p. 137-139.
Beck PR. Butanol extraction of serum and urinary g-glutamyltransferase and its application in clinical diagnosis. Ann Clin Biochem 1978;15:151-156.
Khayrollah AA, Al-Tamer YY, Taka M, Skursky L. Serum alcohol dehydrogenase activity in liver diseases. Ann Clin Biochem 1982;19:35-42.
Fernández MP, Tutor JC, Paz JM. Determinación del ácido D-glucárico urinario por un procedimiento enzimático. Clin Chem Newsletter 1982;2:77-81.
Makki KA, Beetham R, Richens A. Overnigtht urine specimens for the determination of D-glucaric acid excretion in man. Br J Clin Pharmacol 1979;8:183-186.
Nemesánszky E, Lott JA. Gamma-glutamyltransferase and its isoenzymes. Progress and problems. Clin Chem 1985;31:797-803.
Burlina A. Improved method for fractionating g-glutamyltransferase by electrophoresis on cellulose acetate. Clin Chem 1978;24:502-504.
Perucca E, Edges A, Makki KA, Ruprah M, Wilson JF, Richens A. A comparative study of the relative enzyme inducing properties of anticonvulsant drugs in epileptic patients. Br J Clin Pharmacol 1984;18:401-410.
Hermida J, Fernández MP, Tutor JC. Relationship between changes in drug score, D-glucaric acid excretion, and g-glutamyltransferase and b-glucuronidase serum activities during anticonvulsant treatment. Clin Lab 2002;48:115-119.
Ratanasavanh D, Tazi A, Galteau MM, Siest G. Localization of gamma glutamyltransferase in subcellular fractions of rat and rabbit liver: Effect of phenobarbital. Biochem Pharmacol 1979;28:1363-1365.
Saccheti L, Castaldo G, Salvatore F. Electrophoretic behaviour and partial characterization of disease-associated serum forms of gamma-glutamyltransferase. Electrophoresis 1989;10:619-627.
Huseby NE. Multiple forms of serum gamma-glutamyltransferase. Association with lipoproteins. Clin Chim Acta 1982;124:103-112.
Selvaraj P, Rolston DDK, Balasubramanian KA. Separation of hydrophobic and hydrophilic forms of g-glutamyltransferase from human serum by hydrophobic chromatography on phenylsepharose CL-4B. Clin Chim Acta 1984;138:141-149.
Artur Y, Wellman-Bednawska M, Jacquier A, Siest G. Complexes of serum gamma-glutamyltransferase with apolipoproteins and immunoglobulins. Clin Chem 1984;30:361-333.
Seifert J, Vácha J. Depression of microsomal 5´-nucleotidase and cellular ribonucleases in rat liver after phenobarbital administration. Chem Biol Interactions 1970;2:297-307.
Rees GW, Trull AK, Doyle S. Evaluation of an enzyme-immunometric assay for serum a-glutathione S-transferase. Ann Clin Biochem 1995;32:575-583.
Desmet VJ. Drug-induced liver disease: Pathogenic mechanisms and histopathological lesions. Eur J Med 1993;2:36-47.
Sturgill MG, Lambert GH. Xenobiotic-induced hepatotoxicity: mechanisms of liver injury and methods of monitoring hepatic function. Clin Chem 1997;43:1512-1526.
Buehler R, Hess M, Von Wartburg JP. Immunohistochemical localization of human liver alcohol dehydrogenase in liver tissue, cultured fibroblasts, and Hela cells. Am J Pathol 1982;108:89-99.
Hayes PC, Bouchier IAD, Beckett GJ. Glutathione S-transferase in humans in health and disease. Gut 1991;32:813-818.
Ramana KV, Kohli KK. Differential effects of phenobarbitona on the hepatic and renal Glutathione-S-transferases in the rhesus monkey. Indian J Pharmacol 1998;30:34-37.
Matsuka Y, Wang DH, Suganuma N, Imai K, Ikeda S, Takeka K, Kira S. Differential responses of serum gamma-glutamyltransferase to alcohol intake in japanese males. Acta Med Okayama 2003;57:171-78.
Corresponding Author: J. Carlos Tutor, PharmD, PhD, Laboratorio Central. Hospital Clínico Universitario. 15706 Santiago de Compostela Spain. jocatuva@hotmail.com; josecarlostutor@redfarma.org
Published by the Canadian Society for Pharmaceutical Sciences.
Copyright © 1998 by the Canadian Society for Pharmaceutical Sciences.
http://www.ualberta.ca/~csps