J Pharm Pharmaceut Sci (www.ualberta.ca/~csps) 7(2):241-251, 2004
Multicomposite ultrathin capsules for sustained ocular delivery of ciprofloxacin hydrochloride.
Dipankar Bhadra, Girish Gupta, Sulekha Bhadra, R. B. Umamaheshwari, Narendra Jain1
Department of Pharmaceutical Sciences, Dr. H.S. Gour University, Sagar (MP), 470003, IndiaReceived 20 January 2004, Revised 31 May 2004, Accepted 24 June 2004, Published 16July 2004
PDF Version
Abstract
PURPOSE: The present work is intended to develop a sustained bioadhesive drug delivery system for delivery of Ciprofloxacin Hydrochloride in Cul-de-Sac for sustained and effective antimicrobial chemotherapy. For this, ultrathin multicomposite capsular systems were selected. METHODS: Multicomposite ultrathin capsules are molecular assemblies of tailored architecture having layer-by-layer adsorption of oppositely charged macromolecules onto colloidal particles. In the present study colloidal calcium phosphate core and gluateraldehyde fixed RBCs were used as core on which alginate (-vely charged) and polyallylamine hydrochloride (+vely charged) polyelectrolyte coating was deposited alternatively upto 10th layer. The coating in each subsequent layer was determined by changes in zeta potential. Ciprofloxacin hydrochloride was loaded in the capsules by incubation with the capsules suspended in phosphate buffer saline pH 7.4. The cores of the capsules were then removed by treatment with 0.1N HCl for calcium phosphate core and by sodium hypochlorite for RBC cored capsules. The hollow ciprofloxacin HCl loaded capsules were the evaluated in-vitro for pattern of layer-by-layer drug loading, drug release, stability at various temperatures and ionic concentrations and corneal retention. RESULTS: The core removal process was found to have minimal effects on drug loading in capsules. The drug loading was found to be higher for RBC cored hollow capsules and hence release rate was lower as compared to calcium cored hollow capsules. Draize test for corneal irritancy proved that the capsules were not irritating. The capsules were found to deliver the ciprofloxacin in cul-de-sac of rabbit's eyes for prolonged period. CONCLUSION: Based on corneal retention studies and tear drug concentration, the capsules can be considered for suitable and safe use for sustained ocular delivery of drugs.
Introduction
Undulating developments in the field of medical science have revolutionized the research in the field of advanced drug delivery. Since the development of new molecules and to establish their safety and efficacy is a time consuming process, drug delivery system is of particular concern. Continual research is unfolding many potential within these overlapping branches. During last few years, supramolecular chemistry has resulted into the development of many dynamic supramolecular systems (1). While understanding structural and chemical interaction between host-guest systems, self-assembling system is essential for designing molecules that can mimic natural substrate. Recently in various fields of applied physical chemistry, there is an increased interest in supramolecular self-assembling nanostructures such as ultrathin polymer films, surface modified liposome and organic-inorganic composite nanosized materials etc. At present, a variety of materials, such as wide range of synthetic polyelectrolytes, biopolymers, lipids and inorganic particles have successfully been employed to fabricate multilayered films on the flat substrate by taking advantage of electrostatic interaction between oppositely charged species in their stepwise adsorption from an aqueous solution (2-4). The most important discovery in the field of supramolecular science is the development of "self-assembling ultrathin multilayered capsule".
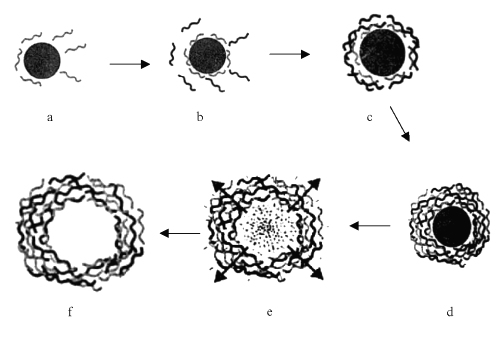
Self-assembling ultrathin multilayered capsule (biomimic capsule) are multilayer films of organic compounds on solid surface and these have been studied for more than 60 years because they allow fabrication of multicomposite molecular assemblies on tailored architecture. However, both the Langmuir-Blodgelt technique and chemiosorption from solution can be used only with certain classes of molecules. An alternative approach for fabrication of multilayers by consecutive adsorption of polyanions and polycations is far more general and has been extended to other materials such as proteins or colloids (2, 5). It is a novel approach to encapsulate various materials and is based on layer-by-layer adsorption of oppositely charged macromolecules onto colloidal particles (6). Different templates with size ranging from 50 nm to few microns, such as organic and inorganic colloidal particles; protein aggregates, biological cells and drug nanocrystals can be coated with multilayered film. Various materials viz. synthetic polyelectrolytes, chitosan and its derivatives, proteins, DNA, lipids, multivalent dyes and magnetic nanoparticles, have been used as layer constituents to fabricate and design shell to adjust required stability, biocompatibility and affinity properties of the capsules (7, 8). Some colloidal templates can be decomposed at conditions where the polymer shell is stable, which leads to the formulation of hollow capsule with defined size, shape and shell thickness (Figure 1).
Figure 1: Schematic illustration of the polyelectrolyte deposition process and of subsequent core decomposition, as a) core attacked by polyelectrolytes; b) coating of charged polyelectrolytes on core; c) coating of alternative charged polyelectrolyte; d) core with well-deposited polyelectrolyte coated layers; e) process of core decomposition and removal from coated polyelectrolytes shells and f) Core removed hollow capsules.
After dissolution of the templating core at low pH, the assembled polyelectrolyte films remain intact (9). The capsule size and wall thickness are determined by the size of the original colloids and the number of adsorbed polyelectrolyte layers, respectively. The permeability through the capsule wall and release of the encapsulated materials depends on the shell wall thickness and composition can be regulated afterwards by pH and ionic strength (10).
The compartmentalization of materials in the small capsule volumes with controlled thickness, composition, and permeability of the encapsulating wall opens perspectives for use of these structures as microreacters, microcarriers, and sustained drug release formulation (11, 12). The fabrication of micro- and nano-sized capsules (or shells) enables the encapsulation of various materials, which are of both scientific and technological interest. Particles embedded in a solid shell (core-shell particles) have been extensively used as microcapsules for the controlled release as studied in the present work and targeting of drugs as well as for the protection of sensitive agents such as cells, enzymes and proteins (13, 14).
Materials and Methods
Materials. Diammonium hydrogen orthophosphate and Poly (allylamine) hydrochloride were purchased from Sigma-Aldrich Co (St. Louis, MO); Sodium hypochlorite, Sodium alginate were purchased from HiMedia Lab, India; and Ca(NO3)2, gluteraldehyde, HPLC water and all other reagents of suitable grades were purchased from CDH, India. Ciprofloxacin hydrochloride was obtained as a generous gift sample from M/S Bro-Shell Remedies Ltd., Sagar (MP), India.
Formulation of capsules using calcium phosphate (dibasic) core. A method for synthesizing core was optimized based on co-precipitation. Water used in the present work is double distilled deionized and membrane filtered HPLC grade water. The process of core formation consists of drop wise addition of 0.19M dm-3 diammonium hydrogen orthophosphate solution to a beaker containing continuously stirred 0.32 M Ca(NO3 )2 solutions at 25°C and controlled pH. During the addition, the pH of Ca(NO3 )2 solutions was maintained at 8-10 using concentrated aqueous ammonia solution. Mixture was then stirred for 1-2 days at the same temperature and pH. The precipitate was filtered, washed thoroughly with distilled water and finally dried at 100oC overnight and used as core.
Ten ml of an aqueous polymer solution of sodium alginate (0.1% w/v) was then added to core particles consisting of 10 ml of aqueous calcium phosphate dispersion (0.2% w/w). Adsorption of polyelectrolyte was allowed for 15 min., with occasional stirring of dispersion. The dispersion was then centrifuged at 2000 rpm for 5 min., the supernatant removed, water added, and the particles re-dispersed by gentle shaking. The centrifugation/wash/re-dispersion cycle was repeated thrice to ensure removal of the polyelectrolyte in solution, generating odd numbered layered capsules. This was followed by deposition of another oppositely charged polyelectrolyte, (poly-allylamine hydrochloride, as 10 ml 0.1% w/v solution) (PAH) generating even numbered layered capsules. This process of coating by sodium alginate/PAH was repeated alternatively up to 10 th layer coating.
After adsorption of desired number of layers of polyelectrolytes and loading of drug, the capsules were exposed to the dil. HCl solution (pH 1.4) for removal of calcium core of capsules. The dil. HCl solution decomposed core calcium phosphate and hence polyelectrolyte hollow capsules containing drug were left behind. The resulted core degradation products and excess HCl were washed off with water and PBS7.4 until a neutral pH of solution was attained on centrifugation/filtration. However, the outermost layer in this study always bears some charge based on the final coat. These capsules are labeled as LBL1.
Formulation of capsules using red blood cell core. Separation of erythrocytes from the whole blood was carried out from the pooled whole blood collected from healthy rabbits in Hi-Anticlot bottle (5 ml) (Himedia Labs., India). To it, normal saline solution was added. The blood was centrifuged at 2500 rpm for 5 minutes. The plasma was pipetted out and buffy coat was carefully removed. The separated RBCs were washed thrice with phosphate buffered saline (PBS), pH 7.4. The washed RBCs were again incubated in 0.06 % of gluteraldehyde (v/v in PBS) for 10 minutes to cause rigidization of the RBCs membrane. The number of RBCs in suspension was determined and adjusted to 100 per ml by 0.9% NaCl and stored at 4°C until used.
Preparation of capsules using RBC core is same as described above for calcium phosphate core except core removal. RBC core was removed by 1.2% v/v sodium hypochlorite at room temperature (25°C). In the case of RBCs, great care has to be taken to prevent aggregation or adhesion by intermittent stirring during the formation of the first four or five layers. These capsules were labeled as LBL2.
Characterization of formulations. Core particles and ultrathin capsules were visualized in an optical microscope (Leica, Germany). A thin film of ultrathin capsules was spread on a slide; a cover slip was placed and observed under the microscope. Photomicrographs were taken at suitable magnifications. The shape and surface topography of ultrathin capsules were also visualized by scanning electron microscopy (SEM), using Leo VP 435 electron microscope. The samples for SEM were prepared by lightly sprinkling the ultrathin capsules on a double adhesive tape, which was struck to an aluminum stub. The stubs were then coated with gold. The samples were then randomly scanned and photomicrographs were taken at different magnifications. The average capsule size and size distribution of formulations were determined in a Laser Diffraction Particle Size Analyzer (CILAS 1064, France), using computerized inspection system. Data of size and size distribution of formulations were also used for optimization of formulations prepared by varying the conditions of the formulations.
Percentage yield was determined using hemocytometry (15). The formulations were diluted twice with distilled water and numbers of capsules per cubic mm were counted by optical microscopy using standard haemocytometer chamber. The capsules in 80 small squares were counted and calculated using the following formula.
Total number of capsules per Cu. Mm = (Total no. of Capsules X Dilutions X 4000)/Total no. of Squares counted (1)
% Yield= (Total no. of hollow capsules per cu. mm/total no of core particles per cu. mm) X 100 (2)
Data of percent yield of different formulations were used in optimization studies. Finally, optimized formulations of calcium phosphate and RBC core were also studied for the layer-by-layer percent yield. Electrophoretic mobilities of the core and coated capsules were measured using a Zetasizer 3000 HS (Malvern, U.K.) to ascertain coating of subsequent oppositely charged layer on capsules. The mobility or velocity of particles v under E as electric field (16) was converted into zeta potential (x) values using the relevant relation (x) = 150v/E. All (x) -potential measurements were performed without added electrolyte in double distilled deionized water of HPLC grade.
Drug entrapment and release studies. For entrapment of drug the prepared capsules formulations were incubated for different time in 10% ciprofloxacin HCl solution in Phosphate Buffer Saline pH 7.4 (PBS7.4). Percent entrapment of drug was determined for various incubation times. Percentage drug entrapment in capsules and effect of core removal on drug entrapment was determined by centrifugation method. The drug loaded capsules having various layer thickness in suspension were centrifuged at 3000 rpm for 5 minutes and supernatant was analyzed for free drug (unentrapped) content spectrophotometrically at 275 nm after suitable dilution with distilled water. Then the drug-loaded capsules were suspended and washed thoroughly three times with distilled water and subjected to core removal using 0.1N HCl, pH 1.4 (for calcium phosphate core) and 1.2% v/v sodium hypochlorite (for RBC core) and again centrifuged at 3000 rpm for 5 minutes. The supernatant of hollow capsules were again analyzed for leached drug. The effect of core removal on drug entrapment efficiency of hollow capsules having various layer thickness was thus determined and compared. These capsules were washed off by PBS pH 7.4, until a neutral pH was established by centrifugation and stored for further studies.
The in vitro drug release profile of entrapped drug from different ultrathin capsule formulations were studied using dialysis tube and artificial cellophane membrane. Two ml of pure and washed capsular suspension free from any unentrapped drug were taken into a dialysis tube and placed in 50 ml of S.T.F. (pH 7.4) and stirred using a magnetic stirrer and the temperature of the assembly was maintained at 37°C throughout the study. Samples were withdrawn at specified time intervals and replaced with the same volume of S.T.F. (pH 7.4). The withdrawn samples were diluted suitably and measured at 275 nm against similarly dialyzed, simple, hollow unloaded capsules, for the drug content. Finally, optimized formulations were studied for layer-by-layer in vitro drug release.
Effect of osmotic pressure on the stability of ultrathin hollow capsules. The ultrathin hollow capsules upon overnight incubation with the solution of electrolytes and non-electrolytes at different concentrations showed drastic changes in the mechanical properties of the system. For that purpose, different concentrations (osmotic pressure) of NaCl and mannitol solutions were used. The initial and final numbers of hollow capsules per cubic mm were counted by optical microscopy using haemocytometer chamber. Initial numbers of intact capsules were taken as 100% for each formulation.
Corneal membrane retention time study (ex vivo). Retention time of the optimized formulations was assessed using the method reported by Mumtaz & Ching, (17), for mucoadhesion with little modification. The eye of she goat was obtained from local slaughterhouse. The corneal membrane of eye was removed and cut in to piece of 16 mm2 and placed in well-aerated Ringer's solution. A strip of corneal membrane was mounted on a glass slide with fixative adhesive. Counted numbers of ultrathin capsules (in 0.1 ml) dispersion was placed on the membrane. This glass slide was incubated for 15 minutes in a desiccator at 90% RH to allow the polymer to interact with the membrane and finally placed in the cell, which was attached to the outer assembly at an angle of 45°. Simulated tear fluid (pH 7.4) was circulated on the cell over the capsules and membrane at the rate of 4 drops per minute from burette. Washings were collected at different time intervals and number of capsules drained off were counted using haemocytometer chamber under optical microscope. Therefore, percent of capsules retained was determined with respect to initial number of capsules incubated.
% Capsules retained = (Initial number of capsules incubated - number of capsules drained off)/Initial number of capsules incubated X 100 (3)
Stability testing. The promising formulations were selected for in vitro stability studies. For that formulations were stored in glass vials at 4±1°C, room temperature (25°C) and at 50°C for 30 days. After 10, 20 and 30 days, they were evaluated for the percent intact capsules left and residual drug content. Percent intact capsules after 10, 20 and 30 days were determined by optical microscopy. Initial percent intact capsules were taken as 100% for each of the formulations.
Entrapment efficiency of stored LBL capsular formulations was determined after 10, 20, 30 days and percent residual drug content was calculated. Entrapment efficiency was determined by the method described earlier. Unentrapped drug was first separated by use of the centrifugation method at 2500 rpm. Then supernatant was measured for drug and remaining amount entrapped in the capsular formulation was determined in Shimadzu UV-1601 spectrophotometer. Initial drug content were taken as 100% for each of the formulations.
Draize test. The animal studies were carried out with the prior permission of CPCSEA. A systemic toxicity study was undertaken taking New Zealand White rabbit as animal of choice as its eyes closely resemble human external eye. Three rabbits were taken at a time for the study of each formulation. They were housed in neat and clean air-conditioned chambers with proper feeding and freedom to relax. Fifty μ l of each formulation (capsules without calcium phosphate core and capsules without RBC core with and without Ciprofloxacin in STF) were instilled into the conjunctival sac of right eye of each rabbit (fixed on rabbit holders for the present study) using micropipette. The lower lid was gently pulled away from cup and formulations were instilled and lids were closed for few seconds. The contra lateral eye served as control with STF instilled in it. The eye was examined after one hour of instillation for any irritation, swelling, redness and lacrimation caused by the formulation under investigation.
Tear drug level studies. Three New Zealand white healthy male rabbits weighing 1.5-2 Kg housed on standard laboratory diet at ambient temperature and humidity in air-conditioned chambers were used for the present studies under strict conditions satisfying experimentation conditions of Institutional Ethical Committee. For studies, rabbits were restrained in normal upright postures in properly designed and comfortable rabbit holders. A single 50 ml dose of formulations containing freeze-dried capsular suspension containing 50 mg of ciprofloxacin suspended in STF pH 7.4 was instilled in right eye using a micropipette inside the center of lower cul-de-sac without actually touching the eyes and irritating the corneal surface.
The same procedure was used to instill simple ciprofloxacin hydrochloride solution in STF and simple STF in contra lateral eye, serving as control.
After a definite period, the tear flowing out of the eyes was collected by closing the eyelids together and sucking out 50 ml of tear using micropipette. The collected tear samples were filtered through a 0.2 mmembrane filter and directly used to determine the drug concentration using HPLC method as suggested by (18) using methanol-0.01M phosphate buffer (18:82) adjusted to pH 2.6 with concentrated orthophosphoric acid as mobile phase, Luna C18 5m column of Phenomenex USA and photo diode array detector SPD-M10A at 275 nm.
Results and Discussion
In recent years, a novel microencapsulation technology based on layer-by-layer assembly of oppositely charged polyelectrolytes has been established (9, 10). In the present study ultrathin capsules were prepared by coating of sodium alginate/PAH multi-layer on a solid core (calcium phosphate or gluteraldehyde fixed RBC) using this technique and subsequently removing the core by decomposition upon exposure to solvent (19). According to the basic concept of the driving force of macro ion multi-layer assembly, the zeta potential of the colloidal particles alternates between positive and negative charges at each step of polyelectrolyte adsorption. The film thickness grows linearly with the number of layers. The increase in thickness was found to be approximately 2 nm/layer of adsorbed polyelectrolyte (10). This technique utilizes the electrostatic attraction and complex formation between polyanions and polycations to form supramolecular multilayer assemblies of polyelectrolytes. Strong electrostatic attraction occurs between a charged surface and an oppositely charged molecule in solution; this phenomenon has long been known to be a factor in the adsorption of small organics and polyelectrolytes (5, 20).
Closer examination of the interactions within these systems has brought out the diversity of structures, film morphology and surface properties achieved by altering shielding and adsorption conditions. As shown schematically in Figure 1, electrostatic interactions between the polyion in the surface are the key to the final structure of the polyion layered thin film. However, secondary, shorter range forces also play a role in determining the film thickness, the final morphology of the film, the surface properties, and can determine whether or not stable multilayer form at all (21). In the present investigation, an attempt was made to embed the broad-spectrum antibacterial, ciprofloxacin HCl in the multilayered polyelectrolyte system to achieve a prolonged action of the drug. Prepared LBL capsules were optimized, characterized and studied for their efficacy for sustained delivery of ciprofloxacin hydrochloride in cul-de-sac of rabbit's eye.
Formulation and characterization of capsules. A drastic increase in average capsule size was observed by increasing the concentration of core from 0.1% w/v to 0.4% w/v of calcium phosphate core and 0.5 ml to 2.0 ml of RBC suspension (100 RBC/ml) in 0.9% w/v NaCl as core. However, the percent yield decreased after 0.2% w/v of calcium phosphate core and 0.5 ml of RBC suspension due to the aggregation of the core particles. So at optimized level, average size of capsules was 7.6 ± 0.5 μm and yield 77.89 ± 0.87 % for LBL1 and 4.5±0.23 μm and 78.9 ± 1.12 % of yield was obtained for RBC cored capsules. The RBC cored capsules were uniform and smaller in shape and size as compared to calcium phosphate and hence more suitable for ocular delivery.
The optimum adsorption time was found to be 15 minutes for both formulations resulting into optimum particle size of 7.6 ± 0.52 mm and 4.5 ± 0.23 mm for LBL1 and LBL2, respectively. On increasing the adsorption time thickness of the polyelectrolyte membrane was drastically increased and surplus polymer-polymer interaction occurred, which resulted in aggregation of capsules. This leads to the lower percent yield of the uniform capsules. Hence, the washing step is necessary just after the optimum adsorption step to remove the surplus amount of polyelectrolyte giving 79.21 ± 0.87 % and 80.12± 0.43 % yield of LBL1 and LBL2 capsules, respectively after the 10th layer of coating. Centrifugation speed is an important parameter in the preparation of ultrathin capsules. In the case of calcium phosphate core and RBC core formulation, optimized speed was found to be 2000 and 2500 rpm, respectively producing average capsule size of 7.6 μm and 4.5 mm for calcium cored and RBC cored capsules, respectively. At lower speed the proper separation of capsules did not occur, some of the capsules present in supernatant were decanted out but at higher speed aggregation occurred which would ultimately affect the percent yield of capsule. In RBC core containing formulations the centrifugation speed was higher because the average size of RBC core is lower in comparison to calcium phosphate core. As well as density of LBL2 are less as compared to calcium phosphate cored which needed increased centrifugation speed producing average yield of 78.02% and 80% for the calcium cored and RBC cored alginate/PAH capsules, respectively.
Dissolution of the core resulted in capsules swelling and breaking due to difference in osmotic pressure. That is why core decomposition is the crucial step in the preparation of these hollow ultrathin hollow capsules. During the core decomposition process, the core was solubilized by lowering the pH to 1.4. The concentration of the ions in degradation products inside capsules becomes quite large. The generated osmotic pressure difference between the bulk and the capsule interior is equilibrated by a hydrostatic pressure difference, the later being responsible for the capsule expansion. The extent and duration of the osmotically induced tension on the capsule wall depends on the interplay between the speed of core dissolution and the permeation rate of the core decomposition products through the capsule walls. Naturally, a larger permeability would be favourable for releasing the tension. In the case of RBC core the percent intact capsule yield (80.18%) obtained was more than calcium phosphate core (62.5%) that is attributed to smaller capsule size (7.5 mm and 4.2 mm for LBL1 and LBL2, respectively) having a higher stability against rupture.
Indeed, at a given pressure difference ∆P the tension in the wall, l, becomes l = ∆Pr/2, where r is the capsule radius. On the other hand, the halftime of the concentration decay is V/AP = r/3P, where P is the wall permeability and V and A are capsule volume and surface area, respectively. Hence, it can be expected that capsules with smaller radii and otherwise the same wall properties should be more stable during the core dissolution process because of Laplace's law, where, when the tension is smaller, and the core degradation product concentration in the interior should decrease faster as a result of the more advantageous ratio between capsule volumes and surface (5). This can also be attributed to the greater free ionic concentration in case of calcium as compared to RBC, which also generated a definite osmotic gradient, moving the solvent flow inside and causing the capsules to burst.
Drug carrier molecule is larger as compared to ions of core (calcium) that was removed by solubilization. The drug molecules also get complexed with the polymer membrane. The complexation is the major hindrance for not losing the drug molecules from the capsule. However, the core in case of the capsules having RBC core (LBL-2) get degraded to smaller simple units, which may remain within the capsules. That may also lead to addition to the steric hindrance preventing drug loss from the capsules. However, the fragments of RBCs so left may not anyhow be immunogenic due to loss of primary structure of larger units of proteins.
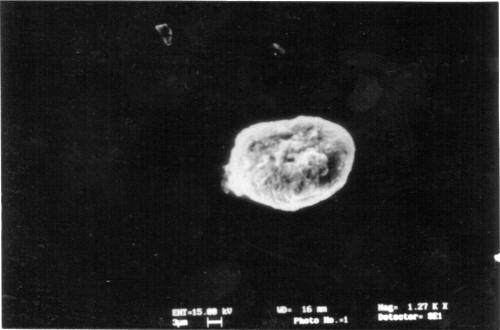
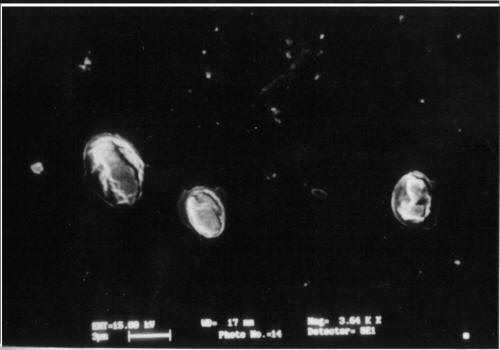
The shape and surface topography of ultrathin hollow capsules were visualized by SEM. The capsules appeared as multilayered structure. Ultrathin capsules were also visualized using optical microscope at every step for determination of aggregation and shape of capsules. The LBL1 capsules are slightly irregular as compared to LBL2 having uniform RBC surface for coating by electrolytes. On removal of core hollow capsules were formed by the adsorption of layers of polyelectrolytes. This is in agreement with the reported studies of polyelectrolyte/dendrimer ultrathin multilayer nanoreservoirs (22). The homogeneous curvature of the capsule proves that, provided the interior aqueous solution has not been removed, the fabricated capsules preserve both the diameter and the spherical shape of the template core (9). The SEM image of the 10 layer (sodium alginate/PAH)5 capsules shows numerous folds and creases attributed to drying of the capsules (Figure 2 & 3).
The capsules also exhibit a somewhat rough surface texture. This may be characteristic of the polyelectrolyte film, although some residual core is present in the capsules. Within the limit of resolution of the SEM technique, no holes or traces of rupture are identified in the capsules. This type of observation was in conformity with the study of Donath et al., (9) for Melamine Formaldehyde core coated capsules of Polystyrene sulphonate/PAH.
Ultrathin capsules were prepared by coating on core (calcium phosphate or RBC) sodium alginate/PAH multilayers by adsorption of charged species, mediated by layer-by-layer assembling protocol (23, 24, 25, 26).
Figure 2: SEM of 10 layered hollow multilayered capsules having Calcium phosphate core.
Figure 3: SEM of 10 layered hollow multilayered capsules having RBC core.
For calcium phosphate core capsules (LBL-1) stepwise growth was confirmed by the successful recharging of the particle surface with each deposition cycle; the zeta potential of the capsules alternated between -19.1 mV (sodium alginate) and +28.6 mV (PAH) with each coating step, suggesting multilayer growth of the particles. No quantitative conclusion can be made from the zeta potential values obtained because the magnitude of the zeta potential is not proportional to the charge density. Since the surface is composed of charges arranged in a layer of finite thickness. Similarly, in the same way for LBL-2, the zeta potential of the capsule alternated between -21.7mV (on sodium alginate coating) and +32.6 mV (on PAH coating) with each coating steps.
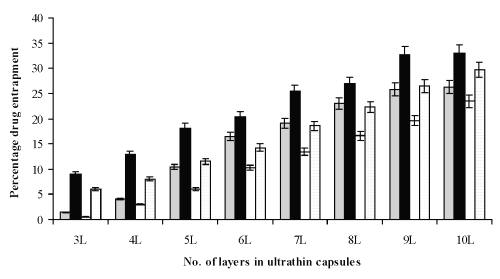
Drug entrapment and release rate studies. The optimized incubation time with maximum drug entrapment is 2 hrs for both formulations. However, a further increase in the incubation time resulted in no change in drug entrapment. The maximum drug loading was found to be 25.7% w/v in hollow capsular suspension (where 1 ml of capsular suspension contains 5000 capsules) in case of calcium phosphate core (concentration of core 2 mg/ml) and 32.2% w/v in case of RBC core (concentration of core 0.5 ml suspension of 100 RBCs in 0.9% NaCl). In case of RBC formulation the drug loading is higher due to the smaller and compact capsule type and higher stability against rupture. Core removal steps affect the drug loading in hollow ultrathin capsules due to rupturing of membrane leading to decrease in entrapment. Layer vs. drug loading study showed that entrapment efficiency has increased as the number of layers increases (Figure 4).
Figure 4: Layer vs. drug entrapment and effect of core removal on drug entrapment of optimized formulations (n=3). Formulations prepared by calcium phosphate core with (
) and without core (
) and Formulations prepared by gluteraldehyde fixed RBC (
) core and without core (
).
The in vitro release of entrapped drug molecules from hollow capsules was found to be concentration-dependent diffusion, in simulated tear fluid (pH 7.4). This illustrates the potential of the sodium alginate/poly(allylamine hydrochloride) based coated systems for the uptake and release of drugs. The in vitro drug release pattern obtained from different ultrathin capsule formulations displayed controlled release profile. Percent cumulative in vitro drug release was found to be 62.43% and 59.63% for hollow LBL1 and LBL2 formulations, respectively (Table 1).
Table 1: Layer-by-layer percent cumulative in vitro drug release from formulation in STF (pH 7.4) (n=3).
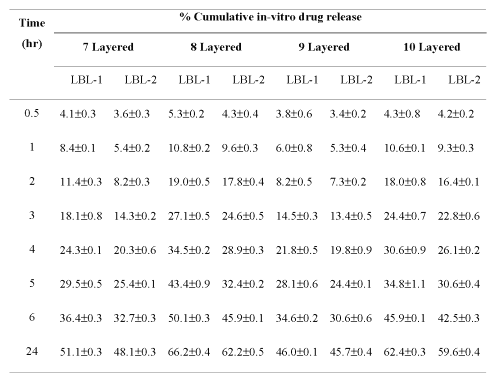
The lesser release rate of drug from hollow LBL2 formulation as compared to hollow LBL1 may be due to the higher affinity of the drug between the uniformly deposited layers of electrolytes on the RBC capsules. The results indicated that hollow LBL2 is a promising controlled release drug delivery system. Layer vs. in vitro release study carried out on the optimized formulations above 7 th layer of polyelectrolytes because in the lower layer there is negligible entrapment of drug. On increasing the number of layers the release rate was slowed down due to the more interaction/entrapment of drug between the polyelectrolyte layers. In both formulations release rate observed for even and odd number of layers, the release rate of ciprofloxacin from odd number of layer (7 th and 9 th ) was slower because of enhanced charge-charge interaction between the drug and polymer (sodium alginate, -ve charge) as compared to even layered (8th and 10th layered) capsules having polyallylamine of positive charges on outermost layers.
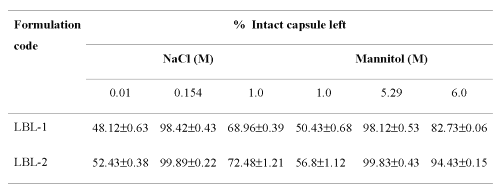
Effect of osmotic strength on capsules .The sodium alginate/PAH capsules are very sensitive to the environmental condition, e.g., temperature, salts (electrolytes and non-electrolytes) and even protein. For the optimal stability of the ultrathin capsules, 0.154 M NaCl and 5.29 M mannitol solution is necessary because these are isotonic to physiologic body fluid. As the osmotic pressure is lowered greater swelling is induced by the osmotic pressure of the sodium counter ions in the capsule interior, which causes burst effect and increase in ionic concentration causes complete shrinking of the hollow capsules, thus ultimately effecting the percent intact capsules (Table 2) suggesting its behaviour as flexible, permeable biological membrane like structure (like biological membrane).
Table 2: Effect of ionic strength on stability of ultrathin hollow capsules (n=3)
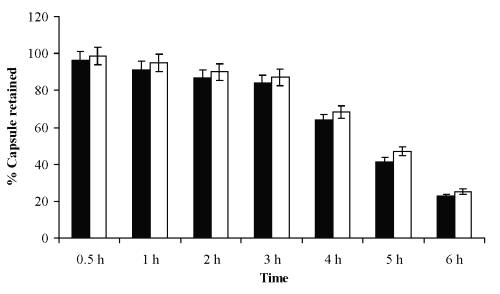
Corneal membrane retention time studies. Retention time study was carried out for measurement of retention of the ten layer coated hollow capsules on the corneal membrane. In both cases results showed that the percent capsule retention was found to be very larger up to 3 hr about 87.06% and 84.06% for LBL-1 and LBL-2 formulations, respectively, after that there is decrease in retention of capsules on membrane but retained up to 6 hr (Figure 5).
Figure 5: Corneal capsular retention time studies of capsules.
This retention is due to charge-charge affinity of positively charged PAH on to negatively charged corneal membrane.
Stability testing studies. The storage stability testing indicated that ultrathin capsular formulation (LBL) stored at 4±1°C was more stable than those stored at room temperature were. Percent intact capsules were found to decrease on storage, which can be attributed to the breaking of the membrane at different storage conditions. This effect was least in the case of formulation stored at 4±1°C, indicating the breaking of the membrane to be a temperature dependent phenomenon and ideal storage condition being 4±1°C (Table 3).
Table 3: Stability testing of multilayered capsules (n=3).
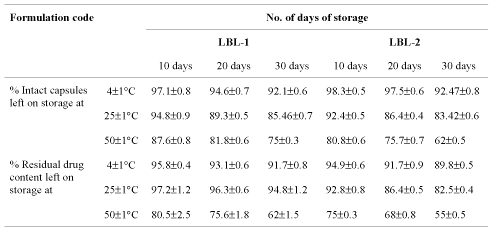
The hollow capsular formulations were stored at 4±1°C, 25±1°C (room temperature) and 50±5°C and the residual drug content measured after 10, 20 and 30 days. The residual drug content of formulation stored at 50±5°C were found to be lowest due to least stability of capsules by polymer solvation at higher temperature than at 25±1°C (room temperature) and highest residual drug content was found in formulations stored at 4±1°C, which indicated that the formulations tend to degrade faster at higher temperature (Table 3).
Draize test .The possible irritancy of the system was ruled out through the Draize test. No sign of swelling and redness was observed in the eye of rabbits during the entire period of study, proving least irritation. However slight to moderate lacrimation was found with hollow types of calcium phosphate and RBC capsular formulations, respectively. This may be attributed to the particulate nature of the formulations not due to irritation. The corneal adhesion can prevent them from being drained out of the sac, hence can possibly deliver drugs in sac for prolonged periods.
Tear drug level studies. The in vivo studies were carried out on male albino rats to determine the corneal drug release profile. Tear from the contra lateral eye was taken as blank to nullify the effects of biological constituents or physiological variations. The results depict that ten-layered hollow LBL2 formulation releases the drug at a slower rate than the LBL1 of similar type. While the conventional formulation of the pure drug showed a maximum drug release within an hour of administration, the system under investigation continued to release the drug at more or less constant rate up to 8 h. Percent cumulative corneal drug concentration was found to have changed during the period of 1 to 8 h for hollow LBL1 (capsules having calcium phosphate core) and hollow LBL2 (capsules having RBC core), which is longer as compared to simple drug solution on instillation (Table 4).
Table 4: Tear level of ciprofloxacin from various formulations (n=3).
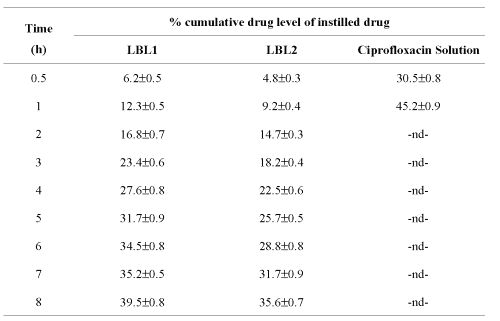
Thus, the results of in vivo data indicated that can be suitable for controlled delivery of the drug to the eye. The results are in conformity with studies of Antipov et al. (2001) for sustained release of Fluorescein from 8-10 layered PSS/PAH Fluorescein cored capsules. However, further investigation would be desirable in order to draw the conclusion for its efficacy.
Conclusion
From all the above studies it can be concluded that these capsular preparations represent interesting, problem free standing systems for loading and controlled release delivery. They can exhibit elastic properties similar to the biological cells, so can be called as "Biomimic Capsules". The prolonged and controlled delivery of drug can be achieved using this system for ocular delivery with reduced drug drainage with the tears as proved by tear drug level studies. The studies showed that the drug release is better sustained by the ten-layered uniformly coated LBL2 capsules (RBC cored) as compared to LBL1 capsules (calcium phosphate cored). However, further animal studies and clinical trials are required to prove efficacy in human volunteers. Similar works on these systems at higher level can definitely be introduced as ocular insert for drug delivery to prolong drug action by minimizing drug loss by overflow from the cul-de-sac.
Acknowledgment
The authors wish to extend their gratitude to Electron Microscopy Div, Dept of Anatomy, AIIMS, New Delhi, India for the SEM studies; Head, Dept. of Pharm. Sc., Dr. H S Gour University, Sagar, MP, India for providing the Lab facilities and UGC, New Delhi, India for funding the research project.
References
Jain, N.K. In: Controlled and novel drug delivery, CBS Publishers, New Delhi, India, 2nd edition, 381-404, 1998.
Decher, G., Fuzzy nanoassemblies: Towards layered polymeric multicomposites. Science, 277, 1232-1237, 1997.
Onda, K., Lvov, Y., Ariga, K. and Kunitake, T., Sequential actions of glucose oxidase and peroxidase in molecular film assembled by layer-by-layer alternate adsorption. Biotech. and Bioengg. 51, 163-167, 1996.
Lvov, Y., Ariga, K., Ichinose, I. and Kunitake, T., Assembly of multicomponent protein films by means of electrostatic layer-by-layer adsorption. J. Am. Chem. Soc. 117, 6117-6123, 1995.
Gao, C., Leporatti, S., Moya, S., Donath, E. and Mohwald, H., Stability and mechanical properties of polyelectrolyte capsules obtained by stepwise assembly of poly (styrene sulphonate sodium salt) and poly (diallyl dimethyl ammonium) chloride onto melamine resin microparticles. Langmuir, 17, 3491-3495, 2001.
Ariga, K., Lvov, Y. and Kunitake, T., Assembling alternate dye-polyion molecular films by electrostatic layer by layer adsorption. J. Am. Chem. Soc. 119, 2224-2231, 1997.
Lvov, Y., Ariga, K., Ichinose, I. and Kunitake, T., Formation of ultrathin multilayer and hydrated gel from montmorillonite and linear polycations. Langmuir, 12, 3038-3044, 1996.
Keller, S. W., Johnson, S. A., Brigham, E. S., Yonomoto, E. H. and Mallouk, T.E., Photoinduced charge separation in multi-layer thin film grown by sequential adsorption of polyelectrolytes. J. Am. Chem. Soc. 117, 12879-12880, 1995.
Donath, E., Sukhorukov, G.B., Caruso, F., Davis, S. A. and Mohwald, H., Novel hollow polymer shells by colloid templated assembly of polyelectrolytes. Angew Chem. Int. Ed. 37, 2202-2205, 1998.
Sukhorukov, G.B., Brumer, M., Donath, E. and Mohwald, H., Hollow polyelectrolyte shells: Exclusion polymers and donnan equilibrium. J. Phys. Chem. B. 103, 6434-6440, 1999.
Dahne, L., Leporatti, S., Donath, E. and Mohwald, H., Fabrication of microreaction cages with tailored properties. J. Am. Chem. Soc. 123, 5431-5436, 2001.
Antipov, A.A., Sukhorukov, G.B., Donath, E. and Mohwald, H., Sustained release properties of polyelectrolyte multilayer capsules. J. Phys. Chem. B. 105, 2281-2284, 2001.
Chia, S. M., Wan, A. C. A., Quek, C. H., Mao, H. Q., Xu, X., Ng, Lu Shen, M. L., Leong, K. W. and Yu, H., Multi-layered microcapsules for cell encapsulation. Biomat. 23(3), 849-856, 2002.
Olga, P. T. and Sukhorukov, G.B., Multilayer alginate/protamine microsized capsules: encapsulation of -chymotrypsin and controlled release study. Int. J. Pharm. 242 (1-2), 155-161, 2002.
Chatterjee, C.C. In: Human physiology, Medical Allied Agency, Calcutta, India, 11th edition, 174, 1985.
Martin, A., Swarbrick, J. and Cammerata, A., Physical Pharmacy, 3rd Ed. (Indian Ed.), Varghese publishing house, Bombay, India, pp. 482-484, 1991.
Mumtaz, A. M. and Ch'ng, H-S., Design of a dissolution apparatus suitable for in situ release study of triamcinolone acetonide from bioadhesive buccal tablets. Int. J. Pharm., 121, 129-139, 1995.
Shafiee, A., Amini, M., Khanavi, M., 2002. Determination of ciprofloxacin in plasma by high performance liquid chromatography. Ind. Drugs. 39(2), 110-112, 2001.
Caruso, F., Caruso, R. A. and Mohwald, H., Nanoengineering of inorganic and hybrid hollow spheres by colloidal templating. Science, 282, 1111-1114, 1998.
Gao, C., Leporatti, S., Moya, S., Donath, E. and Mohwald, H., Surface texture of poly (styrene sulphonate sodium salt) and poly (diallyl dimethyl ammonium chloride) micron sized multiplayer capsules: A scanning force and confocal microscopy studies. J. Phys. Chem. B, 104, 7144-7149, 2000.
Qiu, X., Leporatti, S., Moyer, S., Donath, E. and Mohwald, H., Studies on the drug release properties of polysaccharides multi-layered encapsulated ibuprofen microparticles. Langmuir, 17, 5375-5380.
Khopade, A. and Caruso, F., Electrostatically assembled polyelectrolyte/dendrimer multiplayer films as ultrathin nanoreservoirs. Nano Lett. 2(4), 415-418, 2002.
Voigt, A., Licheten feld, H., Sukhoukov, G.B., Zastrow, H., Donath, E., Baumler, H. And Mohwald, H., Membrane filtration for microencapsulation and microcapsulation formation by layer-by-layer polyelectrolyte adsorption. Ind. Eng. Chem. Res. 38, 4037-4043, 1999.
Murphy, E.F., Lu, J. R., Lewis, A.L., Brewer, J., Russell, J. And Stratford, P., Characterization of protein adsorption at the phosphorylcholine incorporated polymer-water interface. Macromol. 33, 4545-4554, 2000.
Moya, S., Dahne, L., Voigt, A., Leporatti, S., Donath, E., and Mohwald, H., Polyelectrolyte multilayer capsules templated on biological cells: core oxidation influences layer chemistry. Coll. Surf. a- Physicochem. Eng. Aspects, 183, 27–40, 2001.
Neu, B., Voigt, A., Mitlohner, R., Leporatti, S., Gao, C. Y., Donath, E., Kiesewetter, H., Mohwald, H., Meiselman, H. J. and Baumler, H. Biological cells as templates for hollow microcapsules. J. Microencap. 18, 385-395, 2001.
Corresponding Author: Narendra Jain, Department of Pharmaceutical Sciences, Dr. H.S. Gour University, Sagar (MP), 470003, India bhadrasd@sancharnet.in, janrendr@yahoo.co.in
Published by the Canadian Society for Pharmaceutical Sciences.
Copyright © 1998 by the Canadian Society for Pharmaceutical Sciences.
http://www.ualberta.ca/~csps