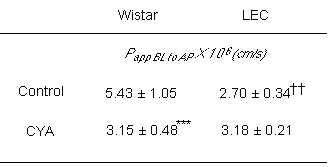J Pharm Pharmaceut Sci (www.ualberta.ca/~csps) 7(2):227-234, 2004
Comparison of urinary excretion of phenolsulfonphthalein in an animal model for Wilson's disease (Long-Evans Cinnamon rats) with that in normal Wistar rats: involvement of primary active organic anion transporter.
Shirou Itagaki, Soji Shimamoto, Takeshi Hirano, Ken Iseki1
Department of Clinical Pharmaceutics & Therapeutics, Graduate School of Pharmaceutical Sciences, Hokkaido University, Kita-ku, Sapporo, JapanMitsuru Sugawara, Sachiho Nishimura, Michio Fujimoto, Michiya Kobayashi, Katsumi Miyazaki
Department of Pharmacy, Hokkaido University Hospital, Kita-ku, Sapporo, JapanReceived 27 April 2004, Revised 21 May 2004, Accepted 25 May 2004, Published 13 July 2004.
PDF Version
Abstract
PURPOSE: The aim of this study was to determine the cause of the decline in phenolsulfonphthalein (PSP) excretion in Long-Evans Cinnamon (LEC) rats. METHODS: The uptake of PSP into rat renal basolateral membrane vesicles (BLMV) was studied. Cyclosporin A (CYA) was used to modulate an ATP-dependent primary active transporter. PSP was intravenously injected into rats with or without CYA. The transcellular transport of PSP was examined by using primary cultured renal proximal tubule cells (PTC). RESULTS: No significant difference was found between the uptake of PSP into renal BLMV of Wistar rats and that into renal BLMV of LEC rats. In the presence of CYA, the urinary excretion and the plasma concentrations of PSP in Wistar rats were decreased and increased, respectively. In primary cultured renal PTC from Wistar rats, the basal-to-apical transport of PSP was greater than that in the opposite direction and the basal-to-apical transport of PSP was substantially reduced by the addition of CYA. However, CYA did not affect the basal-to-apical transport of PSP in PTC from LEC rats. CONCLUSIONS: The results suggest that PSP is transported by primary active organic anion transporter and that the activity level of this transporter is reduced in LEC rats.
Introduction
The Long-Evans Cinnamon (LEC) rat strain is a mutant strain that was established at the Center for Experimental Plants and Animals of Hokkaido University. LEC rats are used as pathological animal models for the study of Wilson's disease (1). In our previous study, the urinary excretion of phenolsulfonphthalein (PSP), a drug that is used for testing renal function because of its high renal clearance (2), after intravenous injection was found to be extremely low in LEC rats (3). However, urinary nitrogen and creatinine excretion in LEC rats did not show serious renal failure. These findings suggested that the function of the secretion system for PSP in the LEC rat kidney is declined. Secretion of organic anions in renal proximal tubules involves the uptake of organic anions across basolateral membranes into cells and exit into the lumen across brush-border (apical) membranes (4). We recently reported that rat organic anion transporter 1 and transporter 3 (rOat1/Slc22a6 and rOat3/Slc22a8) are involved in the renal uptake of PSP and that PSP is a high-affinity substrate for rOat3 but is a relatively low-affinity substrate for rOat1 (5). However, the transporters responsible for the transport of PSP in the brush-border membrane have not been identified. Several ATP-binding cassette (ABC) transporters have been identified in human and rat kidneys (6-8). In the case of excretion from cells into the lumen of the renal tubule, ABC transporters are thought to play an important role as an efflux pump across the brush-border membrane. However, there is little information on the contribution of these efflux transporters to the renal secretion of PSP. Cyclosporin A (CYA) was used in this study to modulate an ATP-dependent primary active transporter, since CYA is known to be an inhibitor of ABC transporters (9).
The purpose of this study was to investigate the role of the renal ATP-dependent efflux transport system in urinary excretion of PSP and to clarify the cause of the decline in PSP excretion in LEC rats.
Materials and Methods
Chemicals
PSP was purchased from Wako Pure Chemical (Osaka, Japan). CYA (Sandimmun®) injections were purchased from a local wholesaler. All other reagents were of the highest grade available and used without further purification.
Animals
Male LEC rats, aged 7 to 8 weeks (200-250 g in weight), were obtained from the Center for Experimental Plants and Animals of Hokkaido University (Sapporo, Japan). Male Wistar rats, aged 7 to 8 weeks (300-350 g in weight), were obtained from NRC Haruna (Gunma, Japan). The housing conditions were described previously (10). The experimental protocols were reviewed and approved by the Hokkaido University Animal Care Committee in accordance with the "Guide for the Care and Use of Laboratory Animals".
Preparation of Basolateral Membrane Vesicles
Renal basolateral membrane vesicles (BLMV) were prepared by self-orienting Percoll-gradient centrifugation with some modification as described previously (5). Kidneys were excised from the rats under sodium pentobarbital anesthesia (40 mg/kg weight, i.p.). Kidney cortex slices (8 to 12 kidneys) were homogenized in ice-cold solution A (300 mM sucrose, 5 mM EGTA, 0.1 mM phenylmethylsulfonyl fluoride and 12 mM Tris / HCl, pH 7.4) with a warning blender at 16,500 rpm for 4 min. The homogenate was rapidly centrifuged at 1,500 x g for 15 min, and the supernatant was recentrifuged at 20,500 x g for 20 min. The fluffy upper-layer pellet was collected and resuspended in 30 ml of buffer with 8% of Percoll and homogenized using a glass Teflon homogenizer with 10 strokes. The crude membrane suspension was centrifuged at 50,000 x g for 60 min. The third fraction from the bottom (approx. 8 ml) was withdrawn and diluted to 32 ml with the buffer. After centrifugation of this fraction at 48,000 x g for 30 min, the pellet on the Percoll solid was collected and resuspended in 20 ml of solution B (100 mM D-mannitol, 100 mM KCl and 20 mM HEPES/Tris, pH 7.5) containing 5 mM EGTA and 10 mM MgCl2. The basolateral membrane was precipitated by centrifugation at 3,400 x g for 15 min. Finally, the pellet was suspended in solution B and recentrifuged at 27,000 x g for 30 min, and the basolateral membrane pellet was resuspended in solution B. Na+-K+ ATPase (a marker enzyme of basolateral membrane) activity of the basolateral membrane was routinely more than 15-fold higher than that of the initial homogenate. In contrast, alkaline phosphatase (a marker enzyme of brush border membrane) activity of the basolateral membrane was routinely the same as that of the initial homogenate. The basolateral membranes were indicated to be enriched at least 15-fold with respect to the brush border membranes.
Uptake experiments
The uptake of substrates into BLMV was measured by the rapid filtration technique described previously (11). In a routine assay, 20 m l of membrane vesicles (0.2-0.3 mg protein) suspension was added to 100 m l of incubation medium kept at 25°C. The compositions on the media are described in the figure legend. At selected time intervals, the uptake was stopped by diluting the incubation medium with 3 ml of ice-cold 10 mM HEPES buffer (pH 7.5) containing 150 mM KCl. The mixture was immediately filtered through a Millipore filter (0.45 m m in pore size, 2.5 cm in diameter; HAWP). The filter was rinsed with 3 ml of the same buffer. Substrate trapped on the filter was extracted with 500 m l of water, and the concentration of the substrate was determined.
Uptake by kidney slices
Uptake studies were carried out as described in our previous report (5). Slices of whole kidneys from LEC rats were put in ice-cold oxygenated incubation buffer. The incubation buffer consisted of 120 mM NaCl, 16.2 mM KCl, 1 mM CaC l2 , 1.2 mM MgSO 4 , and 10 mM NaH 2 PO 4 /Na 2 HPO 4 adjusted to pH 7.5. Slices (80 to 100 mg) were incubated in a 6-well plate with 3 ml of oxygenated incubation buffer in each well after they had been preincubated with incubation buffer for 5 min. The uptake of PSP by kidney slices was measured at PSP concentrations of 25 m M to 1 mM over a period of 30 min at 37°C. After incubation, each slice was immediately removed from the incubation buffer, washed with ice-cold saline, weighed, and homogenized in 0.5 ml saline and the same volume of methanol. After centrifugation (15,000 x g for 15 min) of the mixture, the concentration of PSP in the supernatant was measured.
In vivo study
Four male Wistar rats were used in all experiments. The surgical operation was performed according to a described technique with minor modifications (12). The rats were anesthetized with sodium pentobarbital (40 mg/kg weight, i.p.). The animals were kept on a warm operating table and PSP (0.75 mg/kg) with or without CYA (5 mg/kg), dissolved in saline was injected through the femoral vein of each rat. The volume of drug solution injected into each animal was l00 l. Blood samples were taken from the femoral vein at 1, 15, 30, 45 and 60 min after the injection. Plasma was prepared by centrifugation (850 x g for 15 min) of blood samples. Methanol, corresponding to a double volume of plasma, was added to each plasma specimen. After centrifugation of the mixture (12,000 x g for 15 min), the concentration of substrate in the supernatant was measured. The whole contents of the bladder were withdrawn with a syringe at 60 min after injection.
Primary culture of rat renal proximal tubules cells
Rat renal proximal tubules cells (PTC) were isolated by self-orienting Percoll-gradient centrifugation with minor modification (13). Kidney slices (8 kidneys) were washed 3 times with 10 ml of ice-cold Krebs-Henseleit saline (KHS; pH 7.4) previously gassed at room temperature with 95% O 2 /5% CO 2 for 30 min. The rinsed slices were resuspended in 20 ml of KHS containing 1.5 mg/ml of collagenase (obtained from Clostridium histolyticum ) and 1.0 ml of 5% bovine albumin (fraction V) and stirred for 60 min. The tissue suspension was passed through 40-mesh gauze and centrifuged at 60 x g for 1 min. The tissue pellet was washed 3 times with 10 ml of ice-cold KHS. The washed pellet was resuspended in 20 ml of KHS containing 1.1 mg dispase (obtained from Bacillus polymyxa ) and gently shaken for 1 hr at room temperature. The suspension was centrifuged at 60 x g for 1 min, and the pellet was washed 3 times. The pellet was resuspended in 20 ml of 40% Percoll in KHS and centrifuged at 21,000 x g for 30 min. The proximal tubule fragment was located at the first fraction from the bottom, and the dispased proximal tubule cells were located at the second fraction from the bottom. The cell fraction was collected, washed 3 times with KHS, and suspended in Dulbecco's modified Eagle's medium (not containing phenol red) supplemented with 10% of fetal calf serum with 60 m g/ml of streptomycin and 60 IU/ml of penicillin. For the transport studies, the cells were seeded on polycarbonate membrane filters (3 m m in pore size, growth area of 1.0 cm 2 ) inside Transwell cell culture chambers (Costar, Cambridge, MA) at a cell density of 4 x 10 5 cells/cm 2 . The monolayer cultures were grown in an atmosphere of 95% O 2 /5% CO 2 at 37°C. The medium was renewed every 2 days, and the cells were used on the sixth day after seeding. Only monolayers exhibiting transepithelial electrical resistance (TEER) values > 140 W cm 2 were used.
Transport experiments
Transcellular transport of PSP was measured using monolayer cultures grown in Transwell chambers. For the transport studies, Dulbecco's phosphate-buffered saline (D-PBS) buffer (pH 7.4) containing 137 mM NaCl, 3 mM KCl, 8 mM Na 2 HPO 4 , 1.5 mM KH 2 PO 4 , 1 mM CaCl 2 , 0.5 mM MgCl 2 and 5 mM D-glucose was used. After removal of the culture medium from both sides of the Transwell chamber, the cell monolayers were preincubated with D-PBS (0.5 ml to the apical side and 1.5 ml to the basal side) at 37°C for 20 min. After removal of the medium, the medium on either the basal or the apical side of the monolayers was replaced with a fresh medium containing 30 m M PSP and that on the opposite side was replaced with a fresh medium alone. The monolayers were incubated for up to 60 min at 37°C and gently shaken (100 rpm). Aliquots (each 300 m l) of the incubation medium on the other side were taken at specified times. In the inhibition study, CYA (2 m M) was added to the media on both sides.
Analytical procedures
PSP concentration was determined using an HPLC system equipped with a Hitachi L-6000 pump and L-4200H UV/VIS detector. The column was a Hitachi ODS Gel #3053 (4 mm i.d. x 250 mm). A mobile phase containing 20% acetonitrile and 50 mM H 3 PO 4 with pH adjusted to 3.0 by NaOH was used. Column temperature and flow rate were 55°C and 0.7 ml/min, respectively. Wavelength for detection of PSP was 432 nm. Protein was measured by the method of Lowry et al. (1951) with bovine serum albumin as a standard (14)
Data analysis
Kinetic parameters in the uptake by kidney slices were obtained using the following equation:
v = Vmax1 · S/(Km1 + S)+ Vmax2 · S/(Km2 + S)+Kd· S
where v is the uptake rate of PSP (pmol/min/g kidney), S is the PSP concentration in the medium ( m M), K m is the Michaelis-Menten constant ( m M), and V max is the maximum uptake rate (pmol/min/g kidney). Kd is the rate constant of nonsaturated permeation (nl/min/g kidney). K m1 and V max1 represent the high-affinity component parameter. K m2 and V max2 represent the low-affinity component parameter. The area under the plasma concentration-time curve (AUC) was estimated by the trapezoidal rule using the plasma data from 0 to 60 min. The clearance values of urine (CL urine ) were determined by dividing the amounts excreted into urine from 0 to 60 min by the AUC from 0 to 60 min. The apparent permeability coefficient (P app ) expressed in cm/s was obtained using the following equation:
Papp = dQ/dt · 1/(A · C0),
where dQ/dt is the linear appearance rate of mass in the receiver solution, A is the filter/cell surface area (4.71 cm 3 ), and C 0 is the initial concentration of PSP (30 m M). Student's t-test was used for statistical analysis, and a value of P < 0.05 was considered significant.
Results
Function of rOats in the basolateral membrane of LEC rats
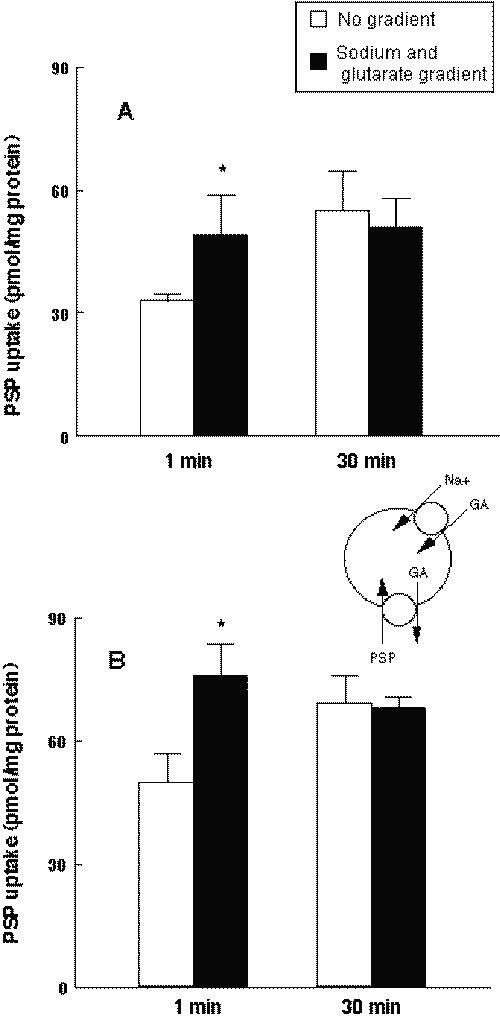
rOat1 and rOat3 have been shown to function as a dicarboxylate-coupled anion exchanger (15, 16). In the BLMV prepared from the Wistar rat, an inward Na + gradient stimulates the glutaric acid (GA) uptake, and this intravesicular GA is exchanged with extravesicular PSP (Figure 1A).
Figure 1: Effects of inward sodium and glutarate gradients on the uptake of PSP by Wistar (A) and LEC (B) rat renal BLMV. Membrane vesicles were suspended in 100 mM D-mannitol, 100 mM KCl and 20 mM HEPES/Tris (pH 7.5). The drug solution contained 100 mM D-mannitol, 0.5 m M glutaric acid, 120 m M PSP, 100 mM KCl or NaCl and 20 mM HEPES/Tris (pH 7.5). Each column represents the mean with S.D. of three preparations. *P < .05, significantly different from no gradient.
The uptake of PSP into the renal BLMV of LEC rats was evaluated. As shown in Figure 1B, the initial uptake of PSP into BLMV of LEC rats was strongly stimulated by the inward Na + and GA gradients.
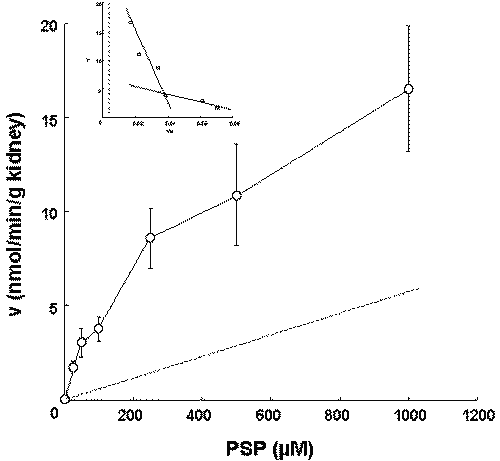
The uptake of PSP by kidney slices prepared from LEC rats were examined (Figure 2).
Figure 2: Concentration dependence of the uptake of PSP by kidney slices in LEC rats. The uptake by kidney slices was measured at PSP concentrations of 25 m M to 1 mM over a period of 30 min. The solid and broken lines represent the fitted line and the uptake corresponding to nonsaturable component, respectively. Each value represents the mean with S.D. of four determinations.
We reported that the uptake of PSP by kidney slices at 30 min was used to examine the concentration-dependence (5). The Km1, Vmax1, Km2, and Vmax2 were determined by kinetic analysis to be 24.3 m M, 2.01nmol/min/g kidney, 896.1 m M and 18.2 nmol/min/g kidney. The profile of the PSP uptake by kidney slices in LEC rats was similar to that in Wistar rats (5).
Inhibitory effect of CYA on the disposition of PSP in Wistar rats
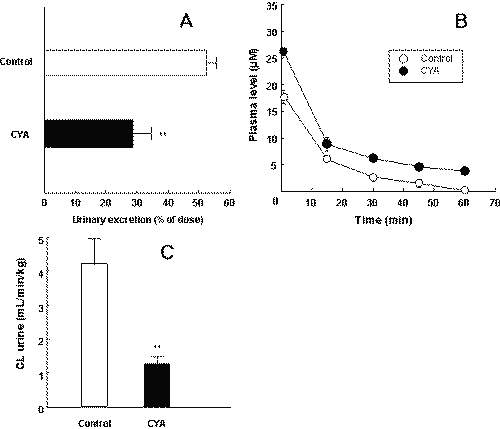
The inhibitory effect of CYA on the excretion of PSP in Wistar rats was examined. The amount of urinary excretion of PSP over a period of 60 min after intravenous injection was determined (Figure 3A).
Figure 3" Effects of CYA on the plasma concentrations (A), urinary excretion (B) and urinary excretion clearance (C) of PSP in Wistar rats. PSP (0.75 mg/kg) was injected through the femoral vein with (closed symbols) or without (open symbols) CYA (5 mg/kg). The time profiles for plasma concentrations and the ratio of the excreted amount of PSP over a period of 60 min in a urine sample to the percent of the injected amount were determined. CL urine values were calculated from the values in Figure 3A and 3B. Each value represents the mean with S.D. of four determinations. *P < .05, significantly different from that in the absence of CYA.
The amount of urinary excretion of PSP was significantly decreased by CYA. The plasma concentration of PSP in the presence of CYA was significantly higher than that in the absence of CYA (Figure 3B).
The calculated AUC value was significantly higher in the presence of CYA. The value of CL urine for PSP in the presence of CYA was significantly lower than the control values (Figure 3C).
Since urinary excretion of PSP was extremely low in LEC rats, the effect of CYA on the excretion of PSP in LEC rats was not determinable.
Transcellular transport of PSP in renal proximal tubule cells
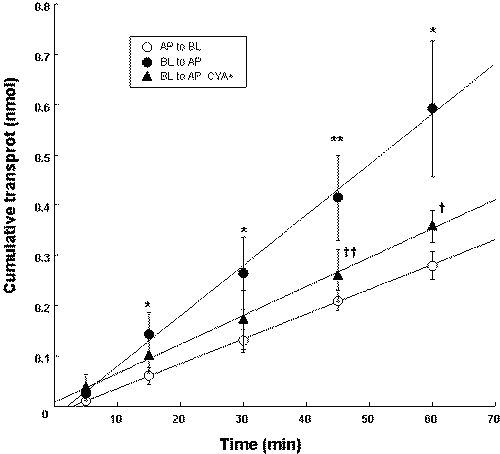
The transcellular transport of PSP in primary cultured renal PTC of Wistar rats was investigated. As shown in Figure 4, the basal-to-apical transport of PSP was greater than the apical-to-basal transport.
Figure 4: Time course of PSP (30 m M) transport across Wistar rat renal PTC monolayers in the presence or absence of 2 m M CYA. Each value represents the mean with S.D. of three determinations. *P < .05, **P < .01, significantly different from apical-to-basal transport. †P < .05, ††P < .01, significantly different from that in the absence of CYA.
In the presence of 2 m M CYA, the basal-to-apical transport of PSP significantly decreased. The transcellular transport of PSP in primary cultured renal PTC of LEC rats was also investigated. As shown in Table 1, the basal-to-apical transport of PSP in LEC rats was significantly lower than that in Wistar rats. CYA had no effect on the basal-to-apical transport of PSP in LEC rats.
Table 1: Effect of CYA on the transepithelial fluxes of PSP across rat renal PTC.
PSP (30 m M) in the sample buffer (control) or in the presence of 2 mM CYA was added to the basal side of primary cultured PTC monolayers. After 60 min at 37°C, PSP transported into apical side was determined and the apparent permeability coefficients were calculated as described in Materials and Methods. Each value represents the mean with S.D. of three to six determinations. ***P < .001, significantly different from that in the absence of CYA. ††P < .01, significantly different from the values in Wistar rats.
Discussion
LEC rats are recognized to be a unique and useful animal model of human Wilson's disease (1). Wilson's disease is an autosomal recessive disorder of copper metabolism characterized by hepatic cirrhosis and neuronal degeneration. The Wilson's disease gene is expressed as a single 7.5-kb transcript in the normal rat liver, and the LEC rat is deficient in this expression (17). LEC rats have defective urinary excretion of PSP, whereas urinary nitrogen and creatinine excretion did not show serious renal failure (3). In the present study, we attempted to clarify the cause of the decline in PSP excretion in LEC rats.
In the first part of this study, we investigated the function of basolateral organic anion transporters in LEC rats. Recently, we have found that rat organic anion transporters are involved in the renal uptake of PSP on the basolateral membrane of proximal tubules (5). Thus, it is possible that dysfunction of these transporters is responsible for the reduction of PSP excretion in LEC rats. No significant difference was found between the initial uptake of PSP into renal BLMV in Wistar rats and that into renal BLMV in LEC rats. Moreover, the profile of the PSP uptake by kidney slices in LEC rats was similar to that in Wistar rats. These results suggest that the functions of organic anion transporters in the renal basolateral membrane are normal in LEC rats.
Because of the limited methodology available for evaluating transport across the brush-border membrane, the excretion mechanism for organic anions on the brush-border membrane of proximal tubules remains to be clarified (18). In the second part of this study, we focused on the excretion system for PSP across brush-border membranes. Several ATP-dependent efflux transporters have been identified in human and rat kidneys (6-8). These transporters confer multidrug resistance against a wide spectrum of cytotoxic agents. We investigated the function of ABC transporters in LEC rats. CYA reduced the CLurine value for PSP in Wistar rats. In primary cultured renal PTC of Wistar rats, the basal-to-apical transport of PSP was greater than that in the opposite direction. Since this basal-to-apical transport was substantially reduced by the addition of CYA, apical-localized ABC transporter(s) may be responsible for the secretion of PSP into the proximal lumen. In the final part of this study, we investigated the function of ABC transporters in LEC rats using primary cultured PTC. The P app BL to AP value of PSP in LEC rats was significantly lower than that in Wistar rats. In contrast to the Papp BL to AP value of PSP in Wistar rats, CYA had no effect on the Papp BL to AP value of PSP in LEC rats. These results suggest that the apical-localized ATP transporter(s) is impaired in LEC rats. Deficient expression of the Wilson's disease gene may affect the function of this ABC transporter(s).
Two ABC transporters for amphipaths, Mdr1 P-glycoprotein (P-gp/Abcb1) and multidrug resistance-associated protein 2 (Mrp2/Abcc2), have been shown to be localized to the renal brush-border membrane (6, 7). Generally, a substrate of P-gp is thought to be a lipophilic and neutral or cationic drug (19). On the other hand, Mrp2 recognizes glucuronide conjugates and non-conjugated organic anions (20). Therefore, it is likely that Mrp2 plays a role in the urinary excretion of PSP because PSP has the structure of an amphipathic anion. However, it has been reported that the transporter function of organic anions on the kidney is maintained in Mrp2 mutant rats (21-23). Therefore, it has been proposed that the contribution of Mrp2 to urinary excretion is minor. Recently, it was found that Mrp4 (Abcc4), another multidrug resistance-associated protein, is localized to the brush-border membrane of renal proximal tubules (8). Mrp4 may be responsible for the urinary excretion of PSP in Wistar rats and the activity level of Mrp4 may be decreased in LEC rats. However, no substrates or inhibitors that are selective for Mrps have yet been found. Molecular analysis is needed to clarify the renal transport system of PSP.
Regulation of the function of transporters should allow efficient development of drugs with ideal pharmacokinetic profiles. As the development of drugs that involves the use of transport mechanisms proceeds, the need for an effective in vitro screening system for transporters will also increase. Accordingly, methods enabling rational prediction and extrapolation of in vivo drug disposition from in vitro data are also essential (24). Although several in vitro models have been used to identify substrates of Mrp2, elucidation of the key in vivo role of Mrp2 in hepatobiliary disposition and elimination of drugs and their conjugates has been facilitated by the discovery of mutant rats with hereditary defect resulting in the loss of expression of functional Mrp2 (25). The complexity introduced by multiple roles of transporters in the elimination of drugs emphasizes the need to have appropriate in vivo models to evaluate the significance of an individual drug transporter in the disposition and elimination of drugs. In the absence of highly specific and potent inhibitors of drug transporters, the use of mutant strains and knockout models is clearly pivotal in the understanding of this role. LEC rats might be useful for clarifying the renal transport mechanism of organic anions.
In summary, the results of this study suggest that the functions of organic anion transporters in the renal basolateral membrane are normal in LEC rats. Moreover, it is possible that the apical-localized ABC transporter(s) actively secretes PSP into the proximal tubule lumen and that the activity level of this transporter is decreased in LEC rats. LEC rats should be useful for clarifying the renal transport mechanism of organic anions.
References
Li, Y., Togashi, Y., Sato, S., Emoto, T., Kang, J. H., Takeichi, N., Kobayashi, H., Kojima, Y., Une, Y. and Uchino, J., Spontaneous hepatic copper accumulation in Long-Evans Cinnamon rats with hereditary hepatitis. A model of Wilson's disease. J Clin Invest, 87:1858-1861, 1991.
Gault, M. H., Koch, B. and Dossetor, J. B., Phenolsulfonphthalein (PSP) in assessment of renal function. J Am Med Ass, 200:871-873, 1967.
Iseki, K., Kobayashi, M., Ohba, A., Miyazaki, K., Li, Y., Togashi, Y. and Takeichi, N., Comparison of disposition behavior and de-coppering effect of triethylenetetramine in animal model for Wilson's disease (Long-Evans Cinnamon rat) with normal Wistar rat. Biopharm Drug Dispos, 13:273-283, 1992.
Inui, K., Masuda, S. and Saito H., Cellular and molecular aspects of drug transport in the kidney. Kidney Int, 58:944-958, 2000.
Itagaki, S., Sugawara, M., Kobayashi, M., Nishimura, S., Fujimoto, M., Miyazaki, K. and Iseki, K., Major role of organic anion transporters in the uptake of phenolsulfonphthalein in the kidney. Eur J Pharmacol, 475:85-92, 2003.
Thiebaut, F., Tsuruo, T., Hamada, H., Gottesman, M. M., Pastan, I. and Willingham, M. C. Cellular localization of the multidrug-resistance gene product P-glycoprotein in normal human tissues. Proc Natl Acad Sci USA, 84:7735-7738, 1987.
Schaub, T. P., Kartenbeck, J., Konig, J., Vogel, O., Witzgall, R., Kriz, W. and Keppler, D., Expression of the conjugate export pump encoded by the mrp2 gene in the apical membrane of kidney proximal tubules. J Am Soc Nephrol, 8:1213-1221, 1997.
van Aubel, R. A., Smeets, P. H., Peters, J. G., Bindels, R. J. and Russel, F. G. M., The MRP4/ABCC4 gene encodes a novel apical organic anion transporter in human kidney proximal tubules: putative efflux pump for urinary cAMP and cGMP. J Am Soc Nephrol, 13:595-603, 2002.
Bohme, M., Buchler, M., Muller, M. and Keppler, D., Differential inhibition by cyclosporins of primary-active ATP-dependent transporters in the hepatocyte canalicular membrane. FEBS Lett, 333:193-196, 1993.
Itoh, T., Itagaki, S., Sasaki, K., Hirano, T., Takemoto, I. and Iseki, K., Pharmacokinetic modulation of irinotecan metabolites by sulfobromophthalein. J Pharm Pharmacol, 56:809-812,2004.
Kobayashi, M., Iseki, K., Saitoh, H. and Miyazaki, K., Uptake characteristics of polyamines into rat intestinal brush-border membrane. Biochim Biophys Acta, 1105:177-183, 1992.
Itoh, T., Takemoto, I., Itagaki, S., Sasaki, K., Hirano, T. and Iseki, K., Biliary excretion of irinotecan and its metabolites. J Pharm Pharmaceut Sci, 7:13-18, 2004.
Vinay, P., Gougoux, A. and Lemieux, G., Isolation of a pure suspension of rat proximal tubules. Am J Physiol, 241:F403-F411, 1983.
Lowry, O. H., Rosebrough, N. J., Farr, A. L. and Randall, R. J., Protein measurement with the folin phenol reagent. J Biol Chem, 193:265-275, 1951.
Shimada, H., Moewes, B. and Burckhardt, G., Indirect coupling to Na+ of p-aminohippuric acid uptake into rat renal basolateral membrane vesicles. Am J Physiol, 253:F795-F801, 1987.
Sweet, D. H., Chan, L. M., Walden, R., Yang, X. P., Miller, D. S. and Pritchard, J. B., Organic anion transporter 3 (Slc22a8) is a dicarboxylate exchanger indirectly coupled to the Na+ gradient. Am J Physiol, 284:F763-F769, 2003.
Yamaguchi, Y., Heiny, M. E., Shimizu, N., Aoki, T. and Gitlin, J. D., Expression of the Wilson disease gene is deficient in the Long-Evans Cinnamon rat. Biochem J, 301:1-4, 1994.
Kusuhara, H. and Sugiyama, Y., Role of transporters in the tissue-selective distribution and elimination of drugs: transporters in the liver, small intestine, brain and kidney. J Control Release, 78:43-54, 2002.
Elbling, L., Berger, W., Weiss, R. M., Printz, D., Fritsch, G. and Micksche, M., A novel bioassay for P-glycoprotein functionality using cytochalasin D. Cytometry, 31:187-198, 1998.
Leier, I., Eisenbeiss, J. H., Cui, Y. and Keppler, D., ATP-dependent para-aminohippurate transport by apical multidrug resistance protein MRP2. Kidney Int, 57:1636-1642, 2000.
Takenaka, O., Horie, T., Suzuki, H. and Sugiyama, Y., Different biliary excretion systems for glucuronide and sulfate of a model compound; study using Eisai@hyperbilirubinemic rats. J Pharmacol Exp Ther, 274:1362-1369, 1995.
Morikawa, A., Goto, Y., Suzuki, H., Hirohashi, T. and Sugiyama, Y., Biliary excretion of 17beta-estradiol 17beta-D-glucuronide is predominantly mediated by cMOAT/MRP2. Pharm Res, 17:546-552, 2000.
Terlouw, S. A., Masereeuw, R., van den Broek, P. H., Notenboom, S. and Russel, F. G. M., Role of multidrug resistance protein 2 (MRP2) in glutathione-bimane efflux from Caco-2 and rat renal proximal tubule cells. Br J Pharmacol, 134:931-938, 2001.
Mizuno, N. and Sugiyama, Y., Drug transporters: their role and importance in the selection and development of new drugs. Drug Metab Pharmacokinet, 17:93-108, 2002.
Ayrton, A. and Morgan, P., Role of transport proteins in drug absorption, distribution and excretion. Xenobiotica, 31:469-497, 2001.
Corresponding Author: Ken Iseki, Department of Clinical Pharmaceutics & Therapeutics, Graduate School of Pharmaceutical Sciences, Hokkaido University, Kita 12-jo, Nishi 6-chome, Kita-ku, Sapporo, 060-0812, Japan. ken-i@pharm.hokudai.ac.jp
Published by the Canadian Society for Pharmaceutical Sciences.
Copyright © 1998 by the Canadian Society for Pharmaceutical Sciences.
http://www.ualberta.ca/~csps