J Pharm Pharmaceut Sci (www.ualberta.ca/~csps) 7(2):265-273, 2004
In vitro activity of commercial valerian root extracts against human cytochrome P450 3A4.
Tania Lefebvre, Brian C. Foster1
Office of Science, Therapeutic Products Directorate, Health Canada, Ottawa, Ontario, CanadaCathy E. Drouin, Anthony Krantis, John T. Arnason, John F. Livesey
Centre for Research in Biopharmaceuticals, University of Ottawa, Ottawa, Ontario, CanadaScott A. Jordan2
Bureau of Chemical Safety, Food Directorate, Health Canada, Ottawa, Ontario, Canada.Received 17 November 2003, Revised 13 May 2004, Accepted 14 May 2004, Published 12 August 2004
PDF Version
Abstract
PURPOSE. Valerian root ( Valeriana officinalis L.) has been used since antiquity as a medicinal herb. Recent studies have found that certain herbal products used concomitantly with conventional therapeutic products can markedly affect drug disposition. METHODS. The in vitro effect of aliquots from 14 commercially available single-entity and blended products containing valerian root on cytochrome P450 CYP3A4-mediated metabolism and P-glycoprotein transport has been determined with aqueous, ethanol and acetonitrile extracts. RESULTS. Hydroxyvalerenic acid, acetoxyvalerenic acid and valerenic acid content was analyzed and wide variation was found between samples and compared to the concentrations noted on the product labels. Valerian extracts from the products tested also exhibited a marked capacity to inhibit cytochrome P450 3A4-mediated metabolism and P-glycoprotein transport based upon the ATPase assay. CONCLUSIONS. There is wide variation between commercially available samples of valerian root. The findings from this study suggest that valerian root may have an initial inhibitory effect when taken with therapeutic products. Further work is warranted to determine whether valerian root can affect other CYP450 isozymes and how the results of this in vitro investigation can be extrapolated to in vivo situations
Introduction
Valerian is a widely used plant-based medicine (1-3). Valerian (Valeriana officinalis L.) native to Europe and Asia is indicated mostly as a mild sedative and as a sleep aid (4). Valerian improves subjective experiences of sleep when taken nightly over one- to two-week periods but long-term safety studies are lacking (5). Valerian is a member of the Valerianaceae family that includes up to 250 species (6). Common species include, but are not limited to, Valeriana wallichii, Valeriana edulis and Valeriana officinalis. The latter is composed of five subspecies and is the species most likely to be commonly used in North America. The parts of the plant used for therapeutic purposes are the roots and the rhizomes. The constituents of Valerian include valepotriates (iridoids), the components of the volatile oil, including monoterpenes and sesquiterpenes (valerenic acids), as well as a number of other constituents (7). Valerian products are usually standardized to valerenic acid or sometimes to valeric acid. The identity of the active component(s) in valerian is still uncertain (8).
Reports of drug interactions with many botanicals, including valerian have been noted in the published literature (9-12). Valepotriates have been shown to prolong the action of barbiturates (13) and intra peritoneal administration of valerenic acid produced a dose-related prolongation of pentobarbital sleeping time in mice (14). Other studies showing interactions between valerian constituents and other drugs include a mixture of valepotriates that partially relieved diazepam withdrawal in mice (15). Further evidence of the effect of altered drug metabolism by valerian comes from rodent studies showing an inhibition of methacetin metabolism with oral administration of some, but not all, valerian constituents (16-17). One clinical case report involved hospitalization due to an intoxication with a mixture of barbital and valerian (18). The potential interaction of valerian and alcohol has often been noted (19); however, this interaction has not always been apparent in human studies (20). Gold et al. (3) identified a series of potential interactions between herbal natural health products (NHPs) including valerian and conventional drug therapy that place older people at risk for an adverse drug event. Valerian may also pose a concern during the perioperative period (1). Dergal et al. (21) determined the range of NHPs and conventional drug therapies used by older adults (aged 65 years and over) attending a memory clinic. Of the 195 patients in the sample, 33 (17%) were 'current users' with 11 potential herb-drug interactions in nine patients including one with valerian and lorazepam.
The therapeutic effects of many herbal medicines have been established; however, definitive pharmacological mechanisms of action on the central nervous system remain to be elucidated for many herbal medications. Although several mechanisms have been identified using purified biomarkers, some studies have provided insufficient information to account for the observed effects of the plant or its extracts. This emphasizes the need to consider the additive and synergistic effects of the multiple constituents (22-23). In addition, components of the plant may act to reduce or antagonize the potential toxicity of other constituents. Interactions with drugs may occur through pharmacokinetic and or pharmacodynamic interactions. All cytochrome P450 (CYP) enzymes exhibit similarity in their structure and general mechanism of action; however, there are significant differences in the detailed activity of individual enzymes as well as in the structures and properties of their active sites. CYP3A4 is the most important isoform in critical tissues, such as hepatic and gastrointestinal tract, responsible for xenobiotic metabolism in humans (21). This enzyme is involved in the biotransformation of over 70% of the drugs in use today (24-25); hence, the possible risk of adverse events due to alterations in this pathway is high.
This study examined whether randomly selected commercial valerian products (capsules, soft gel capsules, caplets, tablets, a tincture and teas) have an affect on the activity of CYP3A4-mediated metabolism. The products were analyzed to determine if they contained authentic valerian marker phytochemicals and the levels of these materials.
MATERIALS AND METHODS
Substrates and Reference Compounds
The capsules, tablets, caplets, soft gels, tincture and the teas were obtained from local commercial outlets but are representative of the North American market. These were all identified with a NHP number and vouchers stored at the University of Ottawa. The moisture content for the capsules, tablets and caplets was determined by drying a known weight of material overnight at 37°C. Dibenzylfluorescein (DBF) and cytochrome P450 CYP3A4 supersomes were obtained from BD GENTEST (Woburn, USA). Hydroxyvalerenic acid (HVA) and valerenic acid (VA) were obtained from Dalton Chemical Laboratories Inc (Toronto, ON). Acetoxyvalerenic acid and hydroxvalerenic acid was obtained from Phytochem, Ichenhausen, Germany. Valerenic acid was also obtained from Extrasynthese, Genay, France. All other chemicals and solvents were of analytical grade.
Stock solutions were prepared at room temperature unless stated otherwise under reduced lighting conditions from the plant material within capsules, tea bags, and whole tablets. Capsules, tablets and caplets were extracted at a concentration of 100 mg/ml, vortexed for 1 min and centrifuged for 15 min at 13,000 rpm. Teas were extracted at a concentration of 25 mg/ml, were polytroned for 1 min, and then centrifuged as above. Soft gels were emptied into a 1.5 ml microfuge tube and 1 ml of solvent was added. The solution was vortexed for 1 min and sonicated for 10 min before being centrifuged as above. The extractions were done in three different solvents: deionized water, 70% ethanol and acetonitrile. All extracts were protected from light and were not kept for more than a day.
Cytochrome P450 Assay Procedure
Aliquots (2ml of organic or 10 ml aqueous) of the valerian root stock solution extracts were tested for their ability to inhibit the metabolism of a CYP3A4 marker substrate using an 200 ml in vitro fluorometric microtiter plate assay (26) based upon the method of Crespi et al. (27). The assays were performed in clear-bottom, opaque-welled microtiter plates (96 well, Corning Costar, model # CS00-3632, Corning, NY). In brief, all control and control-blank wells were balanced to contain an equal volume of 70% ethanol, acetonitrile or deionized water present in the extracts in the test and test-blank wells. All wells had the same amount of NADPH (b-nicotinamide adenine dinucleotide phosphate, reduced form (Sigma Chemical Co., St-Louis, MO) solution; phosphate buffer (KH2 PO4 , pH 7.4 (0.5 M)); DBF and CYP3A4 enzyme. To compensate for intrinsic fluorescence or quenching of the extracts, two additional controls were required: Control-blank and test-blank wells with denatured CYP3A4. Test samples without DBF were used to determine if CYP3A4-mediated metabolism of the extracts affected either fluorescence or quenching. The enzyme stock solution was thawed in a 37°C sand bath. The enzyme was denatured by boiling for 15 min. All solutions were vortexed prior to addition to the wells except the solutions that required preservation of enzymatic activity. These were gently mixed and were sonicated for 2 sec before being added to the wells. The plates were agitated and incubated at 37°C in the plate reader and read after 20 min.
All assays were conducted in triplicate and repeated at least once with a fresh sample. Nelfinavir mesylate (1.8mg/well) was used as a positive control to ensure the consistency of the results. The mean and coefficient of variation of each triplicate was calculated. Then, the difference between the test and the test-blank was divided by the difference between the control and the control-blank to compensate for background noise related to the crude product extracts.
P-glycoprotein Assay Procedure
The P-gp ATPase (BD GENTEST) analysis followed the product insert (28) measuring the orthovanadate-sensitive release of phosphate and ATPase activity using a THERMOmax Microplate Reader. Verapamil (Sigma, lot # 56H0925) was examined as a positive control. All assays were conducted in triplicate and repeated once with a freshly prepared sample.
Valerian Content Analysis
Each sample was weighed (approximately 0.5 g) and extracted three times in 8 ml of 70 % ethanol using sonication for 5 min and then centrifuged at 1000 x g for 5 min. The supernatant was collected after each centrifugation and the final extract volume was adjusted to 25 ml. The extracts were filtered through a 0.22 m PTFE membrane prior to injection. Samples were analyzed for AVA, HVA and VA by HPLC adapted from the method of Bos et al. (29) using a 4 mm LiChrospher 100 RP-18, 75 x 4.6 mm analytical column and 5 m LiChrospher 100 RP-18 guard column (E. Merck, Toronto, Canada) with a 45°C column oven temperature in a Varian Star HPLC system at 225 nm. The mobile phase consisted of A: 25 mM NaH2 PO4 pH 2.95 with H3 PO4 B: acetonitrile. Flow rate 1.2 ml/min in a linear gradient: 40%-70% B in 7 min; 70-85% B in 2 min. Standard curves were generated by HPLC from serial dilution injections of pure standards. HPLC-APCI/MS were performed using an Agilent 1100 series LC/MSD VL with an APCI/MS detector equipped with a Waters YMC ODS-AM column (100 x 2 mm i.d., 3-M particle size). Elution conditions with a mobile phase system of water (solvent A), acetonitrile (solvent B) and methanol (solvent C) as follows: initial conditions 90:5:5 (A: B: C) changing to 65:30:5 in 10 min, then to 5:90:5 at 11 min with a flow rate of 0.5 ml/min. For MS detection, the conditions were: APCI conducted at 350°C, nebulizer pressure 60 psig, nitrogen-drying gas 5 l/min, capillary voltage 3000 V, corona current 15 mA. The MS data was collected in the scan mode. Peak identities in samples were confirmed by relative retention time; spectral analysis collected over the wavelength range 200-350 nm against published spectra and mass spectral analysis.
All assays were performed under gold fluorescent lighting and samples were kept under reduced lighting conditions.
Results
Fourteen products containing valerian root in capsules, soft gel capsules, caplets, tablets, single entity teas and a tincture were tested (Table 1).
Table 1: Product information of the valerian root samples examined in this study. Most products had sufficient label information for use and contraindications, form, unit weight, suggested dose, and other ingredients.


There were marked differences in the information provided by the manufacturers on the product label, making it difficult to compare relative amounts in each product. Daily dose of the tablets, capsules and caplets ranged from 1 to 9 units. Less than half of the product labels stated that the products were standardized to 0.8%. Of these 3 were listed to contain valerenic acid (VA), 2 samples (NHP 4 and 6) reported standardization to valeric acid which is not the industry standard, and 1 mentioned valerenic acids (VAs). The moisture content determined for the capsules, tablets and caplets ranged from 0.1 to 5.5%. The actual NHP 6 product weight content of 324 mg or 394 mg for capsule and content (Table 1) was considerably lower than the 500 mg stated on the product label; three products (NHP 1, 2, and 4) had higher weights but only NHP 1 indicated the presence of excipients. The excipients in these 3 products ranged from 39 to 610 mg accounting for about 10 to 75% of the content weight.
Each product was analyzed to determine content of hydroxyvalerenic acid (HVA), acetoxyvalerenic acid (AVA) and VA (Table 2).
Table 2: Valerenic acids measured in commercial valerian root extracts. The values are given in ppm (mg/g dry weight) and the relative proportions of each (%). Hydroxyvalerenic acid (HVA); acetoxyvalerenic acid (AVA), and valerenic acid (VA).
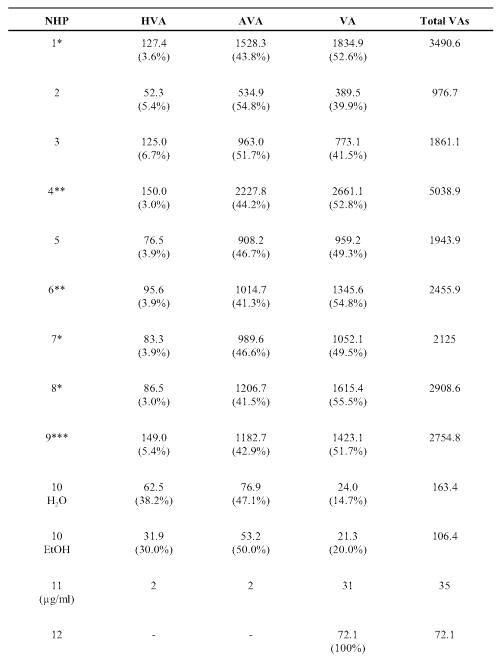
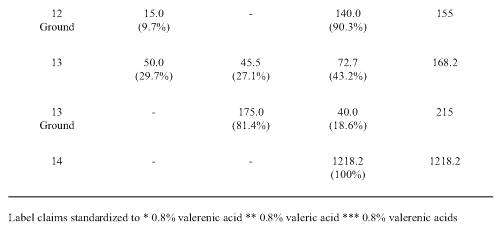
VA was found in all the samples with a wide quantity range from 21 to 2661 ppm. VA account for 14.7-100% of the recovered related acids. The lowest levels were found in the tablet, tincture and two of the teas. The third tea (NHP 14) had relatively higher VA levels. HVA and AVA were not detected beyond traces in 2 of the tea samples (NHP 12 and 14). NHP 4 had the highest total content of 5038.9 ppm valerenic acids. The lowest levels were found in the tablet (NHP 10), the tincture (NHP 11) and in two teas (NHP 109, 142). In most samples, with the exception of NHP 10 and 13, HVA was the minor constituent accounting for less than 10% of the total related acids.
As natural product samples can contain constituents with different extraction coefficients, the effect on CYP3A4-mediated metabolism was determined for three solvent extracts: water, 70% ethanol and acetonitrile (Tables 3, 4 and 5).
Table 3: Percent inhibition of human cytochrome P450 CYP3A4-mediated metabolism by aliquots of valerian root products in capsules extracted in water, 70% ethanol and acetonitrile. Inhibition (at 20 min.) ± Standard deviation (n = 2 in triplicate unless stated otherwise).
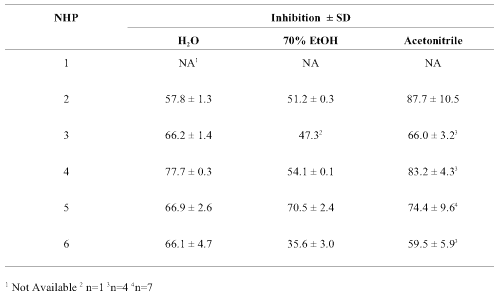
Values obtained with the various capsules (Table 3) were similar with moderate (35-75%) to high (76-88%) inhibition with all samples and extracts. Inhibition of CYP3A4-mediated metabolism tended to be slightly lower in the aqueous extracts and slightly higher in those extracted with acetonitrile. Background fluorescence or quenching was compensated for by the use of appropriate controls and did not change when extracts were incubated with CYP3A4 in the absence of the test substrate DBF.
Results were more variable with the caplet, tablet, tincture and soft gel products examined (Table 4).
Table 4: Percent inhibition of human cytochrome P450 CYP3A4-mediated metabolism by Valerian soft gels, caplets, tablets and tincture extracted in water, ethanol 70 % and acetonitrile. Inhibition (at 20 min.) ± Standard deviation (n = 2 replicates). Inhibition (at 20 min.) ± Standard deviation (n = 2 in triplicate unless stated otherwise).
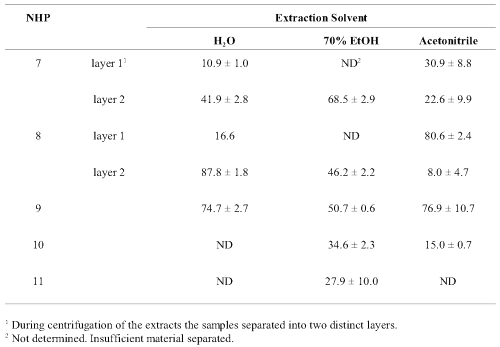
Multiple layers were obtained after centrifugation of the soft gel product extracts. Two distinct liquid layers were obtained with water and acetonitrile. The second layer was very small and insufficiently separated when samples were extracted with ethanol. In the soft gel products, the inhibitory effect varied extensively between products and extraction solvent. Layer 1 of the acetonitrile extracts of NHP 8 gave the highest inhibition, whereas the highest inhibition in the aqueous extract of NHP 8 was found in Layer 2. In each instance, these levels were nearly 2-fold greater than those obtained with NHP 7. The highest levels of inhibition were obtained with the NHP 7 ethanolic extract. The caplet (NHP 9) had moderate activity. Low activity (<35% inhibition) was detected in the tablet (NHP 10) and tincture (NHP 11) products with the highest levels of inhibition in the ethanolic extracts.
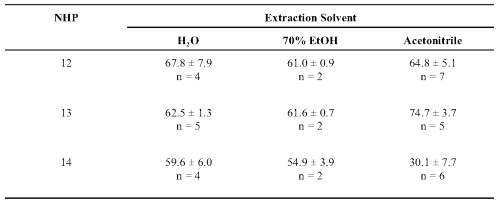
The single-entity teas were initially tested at 100 mg/ml but high levels of inhibition required retesting at 25 mg/ml extracts. The one exception (NHP 14) generally had only moderate inhibitory activity (Table 5).
Table 5: Percent inhibition of human cytochrome P450 CYP3A4-mediated metabolism by extracts of valerian teas. Inhibition (at 20 min.) ± Standard deviation (n = 2-7 replicates).
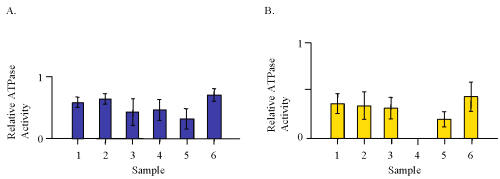
The vanadate-sensitive P-glycoprotein ATPase activity was determined for aqueous and ethanolic extracts of capsule products only (Fig. 1).
Figure 1: ATPase activity for aliquots of 100 mg/ml extracts from capsules containing valerian root expressed as a ratio to 20 mM verapamil from each individual assay. A. Water extracts. B. 70% ethanolic extracts.
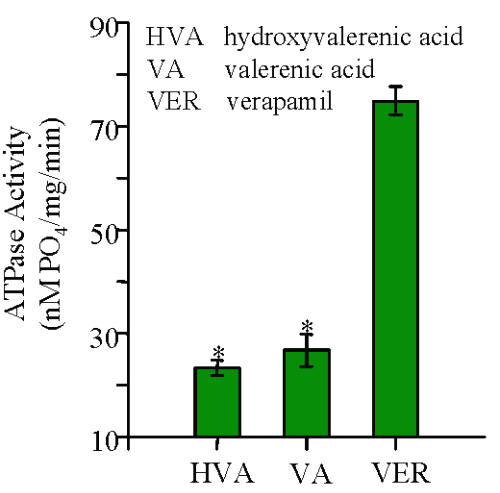
The results expressed relative to 20 mM verapamil show that the water extracts were more active than the 70% ethanolic extracts. The extracts from NHP 6 were the most active effectors in this system. An equivalent amount of authentic reference standards of HVA and VA were examined and found to have similar activity at about 20-25% of that of 20 mM verapamil (Fig. 2).
Figure 2: The vanadate-sensitive P-glycoprotein ATPase activity for 20 μM valerenic acid, hydroxyvalerenic acid and verapamil (n=2).
Discussion
With the increased demand for herbal medicinal preparations, it is increasingly important to elucidate/identify potential adverse effects, including mechanistic interactions between herbal and conventional drugs so that these products can be used safely. The randomly selected samples chosen for this study were found to represent 6 major commercially available categories of valerian products (capsules, tablets, caplets, soft gels, tincture and the teas); and were examined for biomarker content as a surrogate for quality control, and for their ability to affect the major human drug metabolism enzyme and transport protein.
Information printed on some product labels examined in this study stated that the products were standardized to VA, VAs and valeric acid. This is indicative of the confusion created by the industry, as valeric acid is a 5 carbon molecule, which is not related to the much larger VA acids. HPLC analysis of the related VAs revealed all products contained this valerian marker but there was wide variation in content compared to the concentrations stated on the label of some product. With the exception of one tea (NHP 14), the lowest VAs recovery was with tablet, tincture and tea products examined. The highest recovery was observed in the powder capsules. In most samples (NHP 1-9), similar amounts of AVA and VA were present with lesser amounts of HVA. Interestingly two products, the tablet NHP 10 and tea NHP 13 had markedly higher amounts of HVA. The total content of VAs varied from 977 to 5039 ppm and supports the reports of substantial product heterogeneity (29, 30) who found that the concentration of VA and its derivatives ranged from < 0.01 to 6.32 mg/g of product.
Despite the wide variation in actual and reported label weight, and amounts of measured phytochemical constituents, all product extracts were found to have similar moderate to high inhibition of CYP3A4-mediated metabolism and P-glycoprotein ATPase activity. In most cases, the ethanolic extracts were slightly less active than the corresponding water or acetonitrile extracts. There was no apparent relationship or correlation between the amounts of the key HVA, AVA, VA or VAs biomarkers for valerian and this specific biological activity. This finding with CYP3A4 seems to be consistent with Zou et al. (31), who did not find any inhibitory activity with VA. These results are also consistent with our earlier reports with other NHPs (26, 32, 33) and from Williamson (23) that synergistic interactions are important in phytomedicines. The premise being that a whole or partially purified extract of a plant has more pharmacological activity or advantages over a single isolated ingredient. Studies with purified components such as that of Zou et al. (31) can provide information on whether these compounds have an inhibitory effect but negative findings cannot necessarily be extrapolated to the complex phytomedicine. In this instance, standardized valerian root products contain up to 0.8% VA leaving about 99% of the complex mixture to account for the inhibitory effects observed here. The results here show that the VAs are not the only biologically active constituents contained in these natural products which may elicit a systemic or pre-systemic effect on drug disposition. The results here show that the VAs are not the only biologically active constituents contained in these natural products which may elicit a systemic or pre-systemic effect on drug disposition.
There are several confounding factors that make it difficult to infer clinical assessments based on in vitro studies. These can include product heterogeneity resulting from the inherent variability due to harvest, manufacturing processes and the interindividual variability affected by how the product is prepared and consumed. The use of crude extracts presents a second confounding factor in that in situ metabolism of a plant constituent could possibly alter the fluorescence or quenching capacity of the sample resulting in a false result. This can be mitigated somewhat by the use of additional controls but it cannot be eliminated under these assay conditions. With these confounding factors in mind, the in vitro assay can provide some guidance on whether the product may have a qualitative inhibitory effect (34). Ghosal et al. (35) report that the chemical inhibition and correlation data for DBF with CYP3A4 (r = 0.92) indicates that the substrate can be used as specific functional probe for cytochrome P450 3A4. There is no a priori basis to extrapolate either positive or negative in vitro inhibitory results to acute or chronic clinical exposure. However, similar in vitro evidence with extracts from Echinacea (36), garlic (26) and St John's wort (32, 36, 37) predicted a potential pharmacological effect. Clinical studies with Echinacea (38), garlic (39, 40), and St. John's wort (41, 42) have demonstrated that prolonged exposure of these NHPs can have a significant pharmacological effect on drug pharmacokinetics affecting both the safety and efficacy of these drugs. This observation is consistent with that of Liu (43) who documented a number of examples where NHPs were shown to have a biphasic effect on drug metabolism, where administration 1 hr before testing was inhibitory but if administered 24 hr prior to the test they caused induction of drug metabolism. Such biphasic effect has also been noted with many conventional drugs. Little information is reported about the normal use of valerian root or what plasma levels can be obtained for the key biomarkers.
The findings from this study suggest that valerian root may have an initial inhibitory effect when taken with therapeutic products. The variability in the HVA, AVA, VA, total VAs content; and inhibitory effects on CYP3A4-mediated metabolism and ATPase activity suggests that these observations may be similar for other products but caution is warranted if attempting to make such an extrapolation to other lots or formulations of valerian root products. Many consumers of herbal products assume that because the products are natural, there will be no side effects from their use; the evidence presented here adds to the increasing body of evidence suggesting that they are not necessarily biologically benign. Further work is warranted to determine whether valerian root affects other CYP450 isozymes and how the results of this in vitro investigation can be extrapolated to in vivo situations.
Acknowledgements
This research was supported in part by funding from the Positive Action Program, AIDS Bureau of Ontario, AIDS Program Committee of Ontario, and the National Science and Engineering Research Council of Canada (Strategic Program).
References
Ang-Lee, M.K., Moss, J. and Yuan, C.S., Herbal medicines and perioperative care. J Am Med Assoc, 286:208-16, 2001.
Barrett, B., Kiefer, D. and Rabago, D., Assessing the risks and benefits of herbal medicine: an overview of scientific evidence. Alt Ther Health Med, 5:40-49, 1999.
Gold J.L., Laxer D.A., Dergal, J.M., Lanctot, K.L. and Rochon, P.A., Herbal-drug therapy interactions: a focus on dementia. Cur Op Clin Nut Metabol Care, 4:29-34, 2001.
Yager, J., Siegfreid, S.L. and DiMatteo, T.L., Use of alternate remedies by psychiatric patients: illustrative vignettes and a discussion of the issues. Am J Psych, 156:1432-1438, 1999.
Hadley, S. and Petry, J.J., Valerian. Am Fam Phys, 67:1755-1758, 2003.
Foster, S., Valerian. AmeriCanadian Botanical Council. 2000. http://www.herbalgram.org/botanicalbooks/valerian.html.
Bos, R., Woerdenbag, H.J., De Smet, P.A.G.M. and Scheffer, J.J.C., Valeriana Species, in De Smet P.A.G.M., Keller K. and Chandler R.F. (eds), Adverse Effects of Herbal Drugs, Vol. 3. Springer-Verlag, Berlin, pp 165-180, 1997.
Blumenthal, M., Goldberg, A. and Brinkman, J., Herbal Medicine. Expanded Commission E Monographs. Int Med Comm, pp 394-400, 2000.
Brazier, N.C. and Levine, M.A., Drug-herb interaction among commonly used conventional medicines: a compendium for health care professionals. Am J Ther, 10:163-169, 2003.
Huang, S.M., Hall, S.D., Watkins, P., Love, L.A., Serabjit-Singh, C., Betz, J.M., Hoffman, F.A., Honig, P., Coates, P.M., Bull, J., Chen, S.T., Kearns, G.L. and Murray, M.D., Drug interactions with herbal products and grapefruit juice: a conference report. Clin Pharmacol Ther, 75:1-12, 2004.
Izzo, A.A., Drug interactions with St. John's Wort (Hypericum perforatum): a review of the clinical evidence. Int J Clin Pharmacol Ther, 42:139-148, 2004.
Assemi, M., Herbs affecting the central nervous system: gingko, kava, St. John's wort, and valerian. Clin Obs Gyn, 44:824-35, 2001.
Dunayev, V.V., Trzhetsinsky, S.D., Tishkin, V.S., Fursa, N.S., Linenko, V.I. and Stets, V.R., Biological activity of the sum of valepotriates isolated from Valalliariifolia Adams. Farmakol Toksikol, 50:33-37, 1987.
Hendriks, H., Bos, R., Woerdenbag, H.J. and Koster, A.S., Central nervous depressant activity of valerenic acid in the mouse. Planta Med, 1:28-31, 1985.
Andreatini, R. and Leite, J.R., Effect of valepotriates on the behavior of rats in the elevated plus-maze during diazepam withdrawal. Eur J Pharmacol, 269(2-3):233-235, 1994.
Braun, R., Dittmar, W., Hubner, G.E. and Maurer, H.R., In-vivo-Einfluß von Valtrat/Isovaltrat auf Knochenmarkzellen der Maus und auf die Metabolche Aktivitat der Leber. (Influence of valtrate/isovaltrate on the hematopoiesis and metabolic liver activity in mice in vivo). Planta Med, 50:1-4, 1984.
Braun, R., Dieckmann, H., Machut, M., Echarti, C. and Maurer, H.R., Untersuchungen zum Einfluß von Baldrinalen auf hamatopeotische Zellen in vitro, auf die Metabolische Aktivitat der Leber in vivo sowie zum Gehalt in Fertigarzneimitteln (Studies on the effects of baldrinal on hemopoietic cells in vitro, on the metabolic activity of the liver in vivo and on the content in proprietary drugs). Planta Med, 52:446-450, 1986.
Waked, M., Bismuth, C., Capron, F. and Taboulet, P., Lipid pneumonia discovered in a case of epiphenomenal drug poisoning. Presse Med, 20:178, 1991.
Miller, L.G., Herbal Medicinals. Selected clinical considerations focussing on known or potential drug-herb interactions. Arch Int Med, 158:2200-2211, 1998.
Upton, R., Valerian Root (Valeriana officinalis) Analytical, Quality Control, and Therapeutic Monograph, in Upton, R (ed), American Herbal Pharmacopoeia, Santa Cruz, CA, USA, 1999.
Dergal, J.M., Gold, J.L., Laxer, D.A., Lee, M.S., Binns, M.A., Lanctot, K.L., Freedman, M. and Rochon, P.A., Potential interactions between herbal medicines and conventional drug therapies used by older adults attending a memory clinic. Drugs Aging, 19:879-886, 2002.
Spinella, M., The importance of pharmacological synergy in psychoactive herbal medicines. Alt Med Rev, 7:130-137, 2002.
Williamson, E.M., Synergy and other interactions in phytomedicines. Phytomed, 8:401-409, 2001.
SEQ CHAPTER \h \r 1Thummel, K.E. and Wilkinson, G.R., In vitro and in vivo drug interactions involving CYP3A. Ann Rev Pharmacol Toxicol, 38:389-430, 1998.
SEQ CHAPTER \h \r 1Kumar, G.N., Dykstra, J., Robers, E.M., Jayanti, V.K., Hickman, D., Uchic, J., Yao, Y., Surber, B., Thomas, S. and Granneman, G.R., Potent inhibition of the cytochrome P-450 3A-mediated human liver microsomal metabolism of a novel HIV protease inhibitor by ritonavir: A positive drug-drug interaction. Drug Metab Dispos, 27:902-908, 1999.
Foster, B.C., Foster, M.S., Vandenhoek, S., Budzinski, J.W., Gallicano, K.D., Choudri, S., Krantis, A. and Arnason, J.T., An in vitro evaluation of human cytochrome P450 CYP3A4 and P-glycoprotein inhibition by garlic. J Pharm Pharmaceut Sci, 4(2):176-184, 2001.
Crespi, C.L., Miller, V.P. and Penman, B.W., Microtiter plate assays for inhibition of human, drug-metabolizing cytochromes P450. Anals Biochem, 248:188-190, 1997.
GENTEST, Human P-glycoprotein (Pgp or MDR1) Membranes: GENTEST Technical Bulletin, GENTEST Corporation, 1998.
Bos, R., Woerdenbag, H. J., van Putten, F. M., Hendriks, H. and Scheffer, J. J., Seasonal variation of the essential oil, valerenic acid and derivatives, and velopotriates in Valeriana officinalis roots and rhizomes, and the selection of plants suitable for phytomedicines. Planta Med, 64:143-147, 1998.
Shohet, D., Wills, R.B. and Stuart, D.L., Valepotriates and valerenic acids in commercial preparations of valerian available in Australia. Pharmazie, 56:860-863, 2001.
Zou, L., Harkey, M.R. and Henderson, G.L., Effects of herbal components on cDNA-expressed cytochrome P450 enzyme catalytic activity. Life Sci, 71:1579-1589, 2002.
Foster, B.C., Foster, M.S., Vandenhoek, S., Budzinski, J., Krantis, A. and Arnason, J.T., St. John’s wort - effects on cytochrome P450 and P-glycoprotein. Pharmaceut Biol, accepted for publication, 2003.
Foster, B.C., Vandenhoek, S., Hanna, J., Budzinski, J.W., Akhtar, M. H., Bryan, M., Krantis, A. and Arnason, J.T., Effects of natural health products on cytochrome P-450 drug metabolism. Phytomed, SEQ CHAPTER \h \r 110:334-342, 2003.
Tucker, G.T., Houston, J.B. and Huang, S.M., Optimizing drug development: strategies to assess drug metabolism/transporter interaction potential - toward a consensus. Pharmaceut Res, 18:1071-80, 2001.
Ghosal, A., Hapangama, N., Yuan, Y., Lu, X., Horne, D., Patrick, J.E. and Zbaida, S., Rapid determination of enzyme activities of recombinant human cytochromes P450, human liver microsomes and hepatocytes. Biopharm Drug Disposit, 24:375-84, 2003.
Budzinski, J., Foster, B.C., Vandenhoek, S. and Arnason, J., An in vitro evaluation of human cytochrome P450 3A4 inhibition by selected commercial herbal extracts and tinctures. Phytomed, 7:273-282, 2000.
SEQ CHAPTER \h \r 1Obach, R.S., Inhibition of human cytochrome P450 enzymes by constituents of St. John’s wort, an herbal preparation used in the treatment of depression. J Pharmacol Exp Ther, 294: 88-95, 2000.
Gorski, J.C., Huang, S.M., Pinto, A., Hamman, M.A., Hilligoss, J.K., Zaheer, N.A., Desai, M., Miller, M. and Hall, S.D., The effect of echinacea (Echinacea purpurea root) on cytochrome P450 activity in vivo. Clin Pharmacol Ther, 75:89-100, 2004
Gallicano, K., Choudhri, S., Leclaire, T. and Foster, B., Effect of short-term administration of garlic supplements on single-dose ritonavir pharmacokinetics in healthy volunteers, Br J Clin Pharmacol, SEQ CHAPTER \h \r 155:199-202. 2003.
Piscitelli, S.C., Burstein, A.H., Welden, N., Gallicano, K.D. and Falloon, J., The effect of garlic supplements on the pharmacokinetics of saquinavir. Clin Infect Dis, 34:234-8, 2002.
Piscitelli, S.C., Burstein, A.H., Chaitt, D., Alfaro, R.M. and Falloon, J., Indinavir concentrations and St. John’s Wort. Lancet, 355:547-548, 2000.
Markowitz, J.S., Donovan, J.L., DeVane, C.L., Taylor, R.M., Ruan, Y., Wang, J.S., Chavin, K.D., Effect of St John's wort on drug metabolism by induction of cytochrome P450 3A4 enzyme. J Am Med Assoc, 290:1500-1504, 2003
Liu, G., Effects of some compounds isolated from Chinese medicinal herbs on hepatic microsomal cytochrome P-450 and their potential biological consequences. Drug Metab Rev, 23(3&4):439-465, 1991.
Corresponding Author: Brian C. Foster, Health Canada, Therapeutic Products Directorate, Holland Cross 3102C3, 1600 Scott Street, Ottawa, Ontario, Canada, K1A 1B6. brian_foster@hc-sc.gc.ca
2Present Address: Marketed Natural Health Products Division, Marketed Health Directorate, Health Canada, Ottawa, Ontario, Canada.
Published by the Canadian Society for Pharmaceutical Sciences.
Copyright © 1998 by the Canadian Society for Pharmaceutical Sciences.
http://www.ualberta.ca/~csps