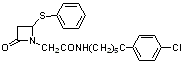J Pharm Pharmaceut Sci (www.ualberta.ca/~csps) 7(1):80-83, 2004
Evaluation of anti-hyperglycemic activity of some novel monocyclic beta lactams.
Rajesh K. Goel1
Pharmacology Division, University Institute of Pharmaceutical Sciences, Panjab University, Chandigarh, India; Department of Pharmaceutical Sciences, Guru Nanak Dev University, Amritsar, IndiaMohinder P. Mahajan
Department of Applied Chemistry, Guru Nanak Dev University, Amritsar, IndiaShrinivas K. Kulkarni
Pharmacology Division, University Institute of Pharmaceutical Sciences, Panjab University, Chandigarh, IndiaReceived 09 December 2003, Revised 16 March 2004, Accepted 29 March 2004, Published 21 April 2004
PDF Version
Abstract
PURPOSE: The present study was undertaken to examine the effect of monocyclic ß-lactams (compounds 5a - 5o) for anti-hyperglycemic activity against alloxan-induced diabetes in rats. As these compounds have been shown to control disturbances in cholesterol metabolism induced by diabetes. METHODS: The test compounds were synthesized via {2+2} cycloaddition (Staudinger) reaction of imines with ketenes. The anti-hyperglycemic effect of test compounds was evaluated in alloxan-induced diabetes in rats by monitoring their effect on blood glucose and liver glycogen contents. RESULTS AND DISCUSSION: In the diabetic rats, high glucose levels and depression in hepatic glycogen contents were observed which could be attributed to the less availability of active form of enzyme glycogen synthetase. Test compounds significantly lowered the serum glucose levels indicating their anti-hyperglycemic activity. This activity of test compounds may be due to increased utilization of glucose as indicated by decreased serum glucose levels and an increase in the activity of glycogen synthetase enzyme as evidenced by rise in liver glycogen contents in test groups. Based on the results structure activity relationship (SAR) has been discussed and favorable substitutions around of monocyclic ß-lactam have been reported. Present study concluded that these compounds could have potential anti-hyperglycemic effect, which might be due to increased utilization of glucose either through increased insulin activity or induction of glycogen synthetase enzyme. Conclusion: Present study concluded that these compounds have significant anti-hyperglycemic effect. Further studies are required to reveal the mechanism of action.
Introduction
Azetidinones have not been reported as exclusive anti-hyperglycemic agents until date. There is a report of compound A of this activity along with other activities such as anti-atherosclerotic, antianginal, antihypertensive etc.1
Compound A
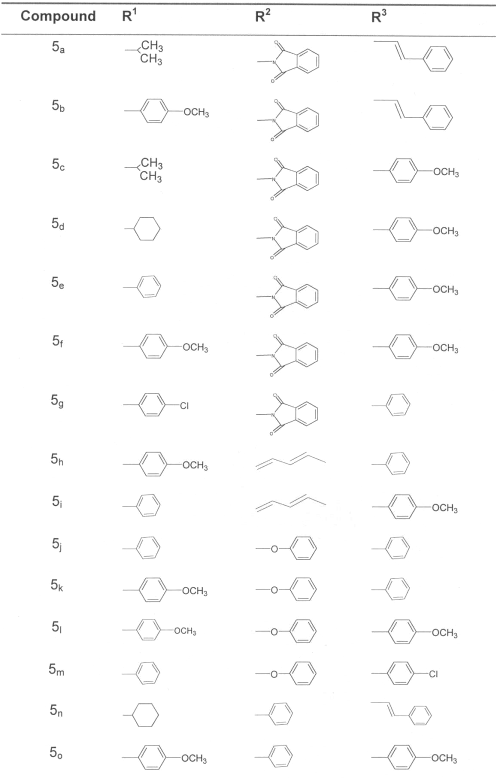
We have evaluated certain novel monocyclic b-lactams for hypocholesterolemic activity in diabetes-induced hypercholesterolemia. These compounds showed their ability to control disturbances in cholesterol metabolism induced by diabetes similar to standard antidiabetic agent gliclazide. These results encouraged us to examine the effect of these compounds on glucose metabolism in diabetes. In the present study compounds 5a - 5o (Table I) were evaluated for their effect on blood glucose and liver glycogen in alloxan-induced diabetes.
Table 1: Substituents of test compounds 5a-50 in 2-Azetidinone ring.
Materials and Methods
Chemistry
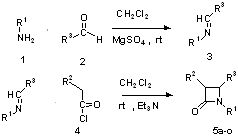
Imines were prepared by condensation of primary amine with equivalent aldehyde proportion in dichloromethane in the presence of anhydrous magnesium sulphate, were treated with ketenes, generated in-situ from acid chlorides in the presence of triethylamine to give desired azetidin-2-ones2 (Scheme-1). Stereochemistry of the synthesized compounds 5a-o (Table I) was assigned based on the reported coupling constant for methine H-3/H-4 protons. ( J = 2-4 Hz and 4-6 Hz for trans-azetidinones and cis-azetidinones respectively)3.
Pharmacology
Animals: Wistar rats of either sex housed in Central Animal House facility of the Guru Nanak Dev University and weighing between 150-200 g were used. The animals were housed under standard laboratory conditions, maintained on a natural light and dark cycle and had free access to food and water. Animals were acclimatized to laboratory conditions before the experimentation. All experiments were carried out between 0900 and 1500 h. The experimental protocols were approved by the Institutional Ethics Committee and conducted according to the Indian National Science Academy Guidelines for the use and care of experimental animals.
Drugs: Alloxan (Loba chemie, Bombay), gliclazide (Panacea Biotec., Lalru (Pb)) , glucose estimation kits (Span diagnostics Ltd., Surat, India) were employed.
Evaluation of antihyperglycemic activity: Diabetes was induced in the animals by single injection of alloxan monohydrate (150 mg/kg, i.p.). It was confirmed after 48 hours (on third day). Animals found hyperglycemic were divided into different groups. Control group received no drug treatment, standard group received gliclazide (8 mg/kg, p.o) and test groups received test compounds 5a-5o(5mg/kg, p.o) once a day up to 14th day of alloxan injection. Blood glucose was estimated in all the groups on 15th day. Animals were sacrificed and their liver tissue was used for the estimation of glycogen.
Estimation of liver glycogen: accurately weighed about 1.0 g of liver tissues, placed the tissues in calibrated centrifuge tube containing 2 ml of KOH (300g /l), and heated in a boiling water bath for 20 min. with occasional shaking. The tubes were cooled in ice, 0.2 ml of saturated sodium sulphate was added, and mixed thoroughly. Then glycogen was precipitated by adding 5 ml of ethanol and the precipitate was removed by centrifugation. The precipitates were dissolved in distilled water (10 ml) with gentle warming. One ml of this solution was added in duplicate in test tubes calibrated at 10 ml, to this 1 ml of HCl (1.2 mol/l) was added After placing marble on the top of each tube, heated in boiling water bath for 2 hours. After 2 hours 1 drop of phenol red indicator was added and neutralized with NaOH (0.5 mol/l). It was diluted to 5 ml with distilled water and the glucose content was determined. Glycogen content was expressed as g / g of liver tissue4.
Statistical analysis: One specific group of rats was assigned to one specific drug treatment condition and each group comprised of six rats (n=6). The data was analyzed using unpaired (2-tailed) Student's "t" test. In all the tests the criterion for the statistical significance was p<0.05.
Results
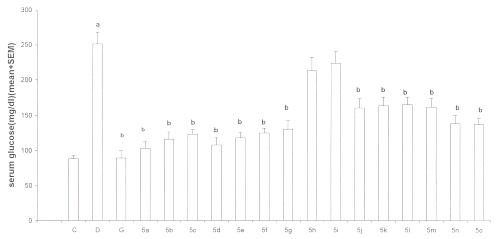
Effect of test compounds on serum glucose levels: The diabetic control group developed significant (p<0.05) hyperglycemia as compared to normal control group. After the treatment with test compounds all the compounds were found to decrease the serum glucose levels significantly (p<0.05) except compounds 5h and 5i when compared with normal control group. However, these compounds showed a lesser activity than gliclazide used as reference standard in the study. The order of anti-hyperglycemic activity for test compounds was found to be 5a>5d>5b>5e>5c>5f>5g>5n>5o>5m>5k>5l>5j. (Figure 1)
Figure 1: Effect of test compounds 5a-5o on serum glucose in alloxan-induced diabetes in rats (n=6-8). C= normal controls D= diabetic controls G= gliclazide treated 5x= test compound treated a=p<0.05 when compared with C and b p<0.05 when compared with D.
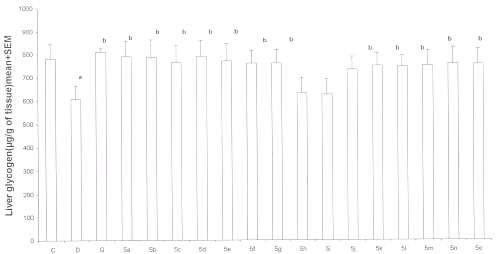
Effect of test compounds on liver glycogen contents: A significant decrease in liver glycogen contents of diabetic controls was observed as compared to normal control group. Test compounds as well as gliclazide prevented the decrease in liver glycogen contents significantly (p<0.05) except compounds 5h and 5i when compared with normal control group. ( Figure 2)
Figure 2: Effect of test compounds 5a-5o on hepatic glycogen content in alloxan-induced diabetes in rats (n=6-8). C= normal controls D= diabetic controls G= gliclazide treated 5x= test compound treated a=p<0.05 when compared with C and b p<0.05 when compared with D.
Discussion
Azetidin-2-ones have been reported as cholesterol absorption inhibitors. However, in this study we have evaluated the compounds (Azetidin-2-ones) for anti-diabetic activity, as these were able to control diabetic hypercholesterolemia. Alloxan a beta-cytotoxin induces chemical diabetes through damage of insulin secreting cells 5 . Induction of diabetes was confirmed by a significant rise in serum glucose and depression in hepatic glycogen contents. Test compounds significantly lowered the serum glucose levels indicating their anti-hyperglycemic activity. In the diabetic rats, high glucose levels and depression in hepatic glycogen contents could be attributed to less availability of active form of enzyme glycogen synthetase, which in turn has been reported to be responsible for incorporation of glucose moieties in pre-existing glycogen chain. 6, 7 This activity of test compounds may be due to increased utilization of glucose as indicated by decreased serum glucose levels and increase in the activity of glycogen synthetase enzyme as evidenced by augmented liver glycogen contents in test groups.
On the basis of order of anti-diabetic activity of test compounds the following structure activity relationship (SAR) was observed
- at C3 phthalmido substitution showed the best activity followed by phenyl and phenoxy substitution.1,3 -butadienyl substitution resulted in total loss of activity.
- at N1 substitution by cyclohexyl and isopropyl favored the activity than phenyl and para-methoxy phenyl.
- at C4 styryl and para-methoxy phenyl substitution was more favorable than phenyl.
The present study on the evaluation of 2-azetidinones as anti-hyperglycemic agents revealed that these compounds are effective as anti-hyperglycemic agents and may act through increased utilization of glucose through either increased utilization of glucose through either either increased utilization of glucose through either either either increased utilization of glucose either through increased insulin activity or induction of glycogen synthetase enzyme.
References
Hickey DMB, Leach CA, Ife RJ and Dhanak D. Azetidinone derivatives for the treatment of atherosclerosis. Chemical Abstracts1998;35313168x
Liebigs S.H. AnnChem 51 (1907)356
Sharma A.K., Mazumdar S.N., Mahajan M.P. J Org Chem 61 (1996)5506- 5509.
Plummer DT. Isolation and assay of glycogen from the liver and skeletal muscles of rats In An introduction to practical biochemistry New York McGraw-Hill1978;182-184
Rerup CC. Drugs producing diabetes through damage of insulin secreting cells Pharmacol Rev 1970; 22:485-520
Goldstein S, Simpson A and Saenger P. Hepatic drug metabolism increase in poorly controlled insulin dependent diabetes mellitus. Acta Endocrinologica 1990;12:3550-558
Mathieu B and Willey S. The hepatic defect in glycogen synthesis in chronic diabetes involves G component of synthase phosphotase. Biochem J 1984;21:7427-435
Corresponding Author: R.K. Goel, Department of Pharmaceutical Sciences, Guru Nanak Dev University, Amritsar 143005, India. goelrk_us@yahoo.comPublished by the Canadian Society for Pharmaceutical Sciences.
Copyright © 1998 by the Canadian Society for Pharmaceutical Sciences.
http://www.ualberta.ca/~csps