J Pharm Pharmaceut Sci (www.ualberta.ca/~csps) 7(1):13-18, 2004
Biliary excretion of irinotecan and its metabolites.
Tatsuya Itoh, Isao Takemoto
Department of Pharmacy, Sapporo Social Insurance General Hospital, Chuo-2-jo, 6-chome, Atsubetsu-ku, Sapporo, JapanShirou Itagaki, Kentaro Sasaki, Takeshi Hirano, and Ken Iseki1
Department of Clinical Pharmaceutics and Therapeutics, Graduate School of Pharmaceutical Sciences, Hokkaido University, Kita-12-jo, Nishi-6-chome, Kita-ku, Sapporo, JapanReceived 07 December 2003, Revised 15 January 2004, Accepted 15 January 2004, Published 23 January 2004
PDF Version
Abstract
PURPOSE: The aim of this study was to investigate the excretion of irinotecan hydrochloride (CPT-11) and its metabolites into the gastrointestinal lumen via the biliary route after intravenous administration of lactone and carboxylate forms of CPT-11. METHODS: Biliary excretions of CPT-11 and its metabolites, SN-38 and SN-38-glucuronide, were investigated by an in vivo administration study using rats. The biliary excretion profiles for both the lactone and carboxylate forms of CPT-11 and its metabolites were determined. RESULTS: After the i.v. injection of the lactone form of CPT-11, the cumulative biliary excretion of SN-38-glucuronide was much greater than that of CPT-11 and SN-38, and biliary excretion of SN-38 was less than that of CPT-11. Further, CPT-11 and SN-38 were mainly excreted into bile as carboxylate forms. After the administration of the CPT-11 carboxylate form, biliary excretion of SN-38-glucuronide was significantly smaller than that after the administration of CPT-11 lactone form. On the other hand, biliary excretion of CPT-11 and SN-38 was greater after dosing with the CPT-11 carboxylate form than that after the CPT-11 lactone form. CONCLUSIONS: The results suggest that the rate of conversion of lactone to carboxylate forms of CPT-11 and its metabolites plays a major role in the biliary excretion of these compounds.
Introduction
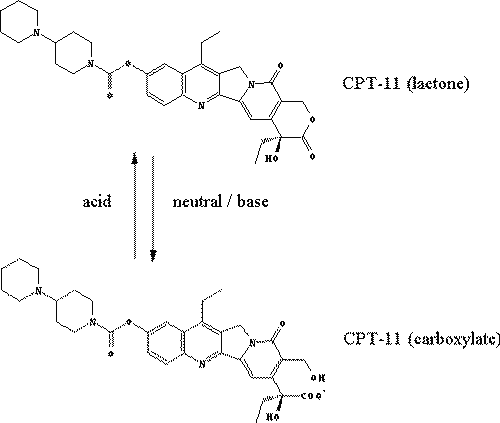
Irinotecan hydrochloride, 7-ethyl-10-(4-[1-piperidino]-1-piperidino)-carbonyloxy-camptothecin (CPT-11), is a synthetic derivative of the plant alkaloid camptothecin, which has demonstrated pronounced antitumor activity. The mechanism of action of CPT-11 involves inhibition of mammalian DNA-topoisomerase-I, causing stabilization of cleavable complexes during DNA replication transcription and repair, and, ultimately, cell death (1, 2). CPT-11 is converted into its active metabolite, 7-ethyl-10-hydroxycamptothecin (SN-38), by carboxylesterase in the liver (3). SN-38 has strong anti-tumor activity. However, SN-38 is not only associated with antitumor activity of CPT-11 but also with CPT-11-induced toxicity (4). The major dose-limiting toxicity after administration of CPT-11 is severe diarrhea that is often unresponsive to common antidiarrheal agents (5). SN-38 is further metabolized by UDP glucuronosyltransferase 1A1 (UGT1A1) to its inactive form, SN-38 glucuronide (SN-38-Glu), and subsequently secreted into the intestine via bile (6). Some studies have shown a correlation between late-onset diarrhea and biliary secretion of SN-38 (7). It has been suggested that beta-glucuronidase derived from enterobacteria may play a major role in the development of CPT-11-induced diarrhea by mediating hydrolysis of SN-38-Glu to form the active SN-38 (8). Therefore, SN-38-Glu is thought to be associated with the severe diarrhea occurring after irinotecan therapy because of the enteric injury caused by SN-38 (9). Theoretically, modulation of CPT-11-induced delayed-type diarrhea by coadministration of an inhibitor of beta-glucuronidase can be advantageous (4). Sodium bicarbonate is used to prevent diarrhea because it inhibits absorption of SN-38 by alkalization of the small intestine (10). All of these agents work intraluminally. However, intestinal toxicity following CPT-11 administration is due to the biliary excretion of SN-38 and SN-38-Glu. It has been reported that cyclosporin A inhibits P-glycoprotein (P-gp/Abcb1) -mediated biliary excretion of CPT-11 and its metabolites and causes dramatic increases in blood concentration of these compounds (11). Generally, a substrate of P-gp is thought to be a lipophilic and neutral or cationic drug (12). However, blood pH (approx 7.4)-dependent hydration converts lactone-form compounds into carboxylate-form compounds (Figure 1).
Figure 1: Hydrolysis and lactonization of CPT-11.
Multidrug resistance associated protein 2 (Mrp2/Abcg2) recognizes glucuronide conjugates and non-conjugated organic anions (13). We focused on these hepatic organic anion transporters because SN-38-Glu and carboxylate forms of both CPT-11 and SN-38 are substrates of Mrp2 (14). In the present study, we investigated the effect of the chemical formation of CPT-11 on the biliary excretion of CPT-11 and its metabolites using in vivo administration. We compared the biliary excretion of CPT-11 and its metabolites after intravenous administration of CPT-11 lactone form and that of carboxylate form.
Materials and Methods
Chemicals
All chemicals and reagents used were of analytical grade. Both CPT-11 and SN-38 were kindly donated by Daiichi Pharmaceutical (Tokyo, Japan). Camptothecin and beta-glucuronidase (from E. coil) were purchased from Sigma Chemical Co. (St Louis, MO). SN-38 was dissolved in DMSO (2% w/v final concentration) due to its hydrophobic property and poor solubility in water. The other compounds were dissolved in distilled water. For preparation of lactone and carboxylate forms of CPT-11 or SN-38, each compound was dissolved in 10 mM potassium phosphate (pH 2.5 and 9.0, respectively) and the sample was left overnight.
Animals
Male Wistar rats, aged 6 to 7 weeks (180-230 g in weight), were obtained from Japan SLC (Hamamatsu Japan). Animals were used without fasting before all experiments. The experimental protocols were reviewed and approved by the Hokkaido University Animal Care Committee in accordance with the "Guide for the Care and Use of Laboratory Animals".
Disposition Studies
Experiments on excretion of CPT-11 were performed in rats in which a cannula (Intramedic PE-50; Clay Adams, Parsippany, NJ) had been implanted into the bile duct under ether anesthesia. The animals were kept in a warm operating table, and CPT-11 (100 mg/body) was administered through the femoral vein. The volume of drug solution injected into each animal was l ml. Bile samples were collected at specified times and prepared immediately.
Preparation of Samples
For determination of lactone forms of CPT-11 and SN-38, bile (40 ml) was mixed with 20 ml of DMSO, 20 ml of diluted water, 40 ml of 2 mg/ml camptothecin as an internal standard, and 0.4 ml of diethyl ether and then vortexed (3 min). After centrifugation of the mixture (5,000 X g for 2 min), the upper aqueous layer was transferred to a microtube and evaporated to dryness. Two hundred ml of 50 mM of monobasic potassium phosphate (pH 2.5) was added to the tube. For determination of total forms of CPT-11 and SN-38, bile (40 ml) was mixed with 0.4 ml of 1 M aqueous zinc sulfate/methanol/ethylene glycol (1/1/2, v/v), 20 ml of DMSO, 20 ml of diluted water and 40 l of 2 mg/ml camptothecin and then vortexed (30 sec). After centrifugation of the mixture (5,000 X g for 2 min), 100 ml of the upper aqueous layer was transferred to a microtube. One hundred ml of 50 mM of monobasic potassium phosphate (pH 2.5) was added. After vortexing briefly, the sample was left overnight at 37°C. SN-38-Glu was determined as SN-38 by the same manner, except that the addition of diluted water containing 10000 U/ml beta-glucuronidase and the sample was left 3 hour at 37°C, to ensure complete deconjugation of SN-38-Glu.
Analytical Procedures
An HPLC equipped with a fluorescence detector was used to determine the lactone and carboxylate forms of both CPT-11 and SN-38 in the bile according to a described technique (15). The column was a C8 column (250 x 4.5 mm, 5 mm; GL Sciences). A mobile phase consisting of [50 mM monobasic potassium phosphate (pH 2.5), 7 mM tetrabutylammonium bromide]: acetonitrile (70: 30, v/v) was used. Column temperature and flow rate were 40°C and 0.8 mL/min, respectively. The fluorescence detector (F1000; Hitachi) was operated at excitation and emission wavelengths of 355 nm and 515 nm, respectively, which yielded the optimum signal-to-noise ratio for all compounds.
Statistics
Student's t-test was used for statistical analysis, and a value of P < 0.05 was considered significant.
Results
Biliary excretion of CPT-11 and its metabolites after administration of the lactone form of CPT-11.
Biliary excretion of CPT-11, SN-38 and SN-38-Glu after administration of the lactone form of CPT-11 was investigated.
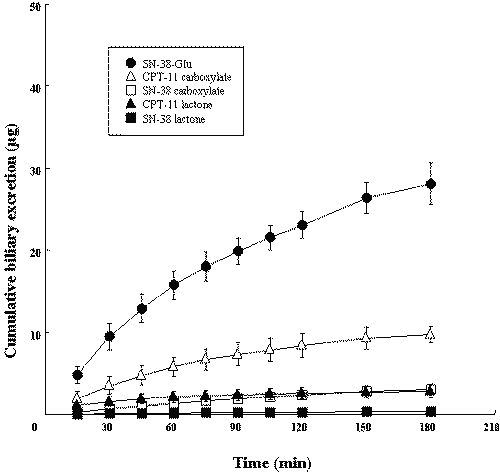
As shown in Figure 2, the cumulative amount of SN-38-Glu excreted into bile over a period of 3 hr after i.v. injection of the lactone form of CPT-11 was much greater than the cumulative amounts of CPT-11 and SN-38, and the cumulative biliary excretion of SN-38 was less than that of CPT-11.
Figure 2: Time profiles for cumulative biliary excretion of CPT-11 and its metabolites after i.v. administration of the lactone form of CPT-11 into rats. The time profiles for cumulative biliary excretions were determined after i.v. injection of the lactone form of CPT-11 (100 mg/body). Each value is the mean with S.D. of three determinations.
It has been reported that Mrp2 recognizes glucuronide conjugates and non-conjugated organic anions. We compared the biliary excretion of lactone forms with that of carboxylate forms. CPT-11 and SN-38 were mainly excreted into bile as carboxylate forms.
Biliary excretion of CPT-11 and its metabolites after administration of the carboxylate form of CPT-11.
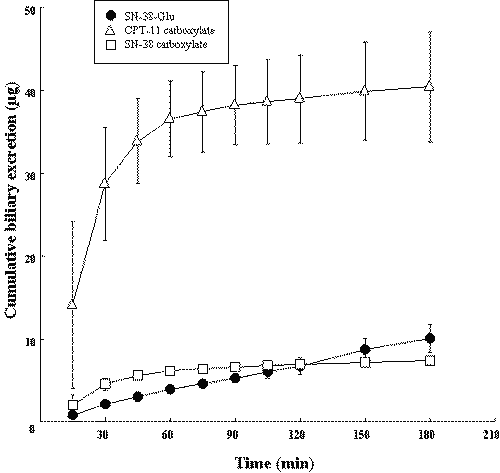
Most of pharmacokinetic and pharmacodynamic studies on CPT-11 have been performed only by administration of the active lactone form (16-18). We investigated the biliary excretion of CPT-11, SN-38 and SN-38-Glu after administration of the carboxylate form of CPT-11. In this experiment, lactone forms of CPT-11 and SN-38 could not be detected in bile samples. Unlike that in the case of administration of the lactone form of CPT-11, biliary excretion of CPT-11 was much greater than that of both SN-38-Glu and SN-38 (Figure 3).
Figure 3: Time profiles for cumulative biliary excretion of CPT-11 and its metabolites after i.v. administration of the carboxylate form of CPT-11 into rats. The time profiles for cumulative biliary excretions were determined after i.v. injection of the carboxylate form of CPT-11 (100 mg/body). Each value is the mean with S.D. of three determinations.
The amount of biliary excretion of SN-38-Glu in dosing with the CPT-11 carboxylate form was significantly smaller than that with its lactone form (Table 1).
Table 1: Comparison of biliary excretion of CPT-11 and its metabolites after i.v. administrations of CPT-11 lactone and carboxylate forms (100 mg/body) in rats.
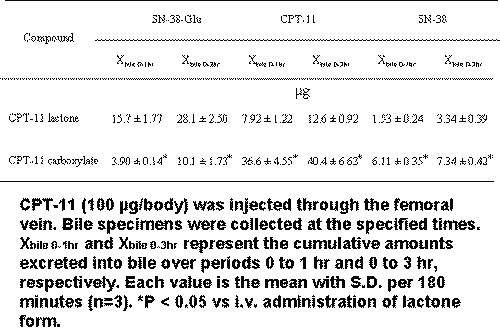
Cumulative biliary excretion of CPT-11 and SN-38 are shown in the total values in Table 1. On the other hand, biliary excretion of CPT-11 and SN-38 was greater in dosing with the CPT-11 carboxylate form than that with its lactone form. During the first 1 hr, large amounts of both CPT-11 and SN-38 were excreted into bile after i.v. dosing with the CPT-11 carboxylate form.
Discussion
Although CPT-11 is a potent and novel anticancer drug, severe side diarrhea has been observed as a side effect in some patients during its clinical use (1-5). To understand the mechanism of this side effect, it is important to clarify the pharmacokinetics of CPT-11. It has been reported that CPT-11 and its metabolites are mainly excreted via biliary route (6). CPT-11 is converted into SN-38, an active metabolite, in the liver. SN-38 is further conjugated to inactive SN-38-Glu and subsequently secreted into bile. It has been reported that hydrolysis of SN-38-Glu into SN-38 by enterobacteria may play a major role in the development of CPT-11-induced diarrhea (8, 9). The purpose of this study was to clarify the mechanism of biliary excretion of CPT-11 and its metabolites with the aim of finding a means to reduce the side effects associated with CPT-11 administration.
After dosing with the lactone form of CPT-11, biliary excretion of SN-38-Glu was much greater than that of CPT-11 or SN-38. It has been reported that Mrp2 is responsible for the biliary excretion of CPT-11 and its metabolites and that Mrp2 has higher affinity for SN-38-Glu than for CPT-11 and SN-38 (14, 19). These reported findings support our results.
Mrp2 recognizes glucuronide conjugates and non-conjugated organic anions (13). At blood pH (approx 7.4), the equilibrium favors hydrolysis to open the lactone ring and yield the carboxylate form (20). The hydrolysis reaction of CPT-11 lactate is more than 10-fold faster than the lactonization reaction of CPT-11 carboxylate. In addition, hydrolysis of lactone rings proceeded under the condition of alkalescent bile. Since SN-38-Glu and the carboxylate forms of CPT-11 and SN-38 have organic charges, we hypothesized that the biliary excretion of these anionic compounds plays a major role in the biliary excretion of CPT-11 and its metabolites. Therefore, we determined the biliary excretion profiles of individual carboxylate and lactone forms of CPT-11 and SN-38. CPT-11 and SN-38 were mainly excreted into bile as carboxylate forms. These results indicate that most of SN-38 and CPT-11 is excreted through the canalicular membrane as two types of hydrophilic anion created by hydration of the lactone ring or glucuronidation of the 10-hydroxyl group.
The antitumor activities of the carboxylate form of CPT-11 and its derivatives have been shown to be weaker than those of the lactone form (21). However, most of pharmacokinetic and pharmacodynamic studies have been performed only by administration of the active lactone form (16-18). In this study, we focused on the pharmacokinetic behaviors of the carboxylate forms of CPT-11 and SN-38. At the end of this study, we used the carboxylate form of CPT-11 as a model compound to reduce the contribution of the hydrolysis reaction of the lactone form of CPT-11. After administration of the carboxylate form of CPT-11, biliary excretion of CPT-11 was much greater than that of SN-38-Glu or SN-38. The present study showed that carboxylate forms of CPT-11 and SN-38 were excreted into bile more rapidly than were the lactone forms. Moreover, it has been reported that the carboxylate forms of CPT-11 and SN-38 have a higher affinity for Mrp2 than do the lactone forms (14, 19). These results suggest that administration of the carboxylate form of CPT-11 accelerates the active excretion of the carboxylate forms into bile by Mrp2 before converting into lactone form. On the other hand, biliary excretion of SN-38-Glu after dosing with the carboxylate form of CPT-11 was significantly less than that after dosing with the lactone form of CPT-11. The conjugation of SN-38 to SN-38-Glu by UGT1A1 was considered to be decreased due to the rapid excretion of the carboxylate forms of SN-38 and CPT-11.
In this study, we demonstrated that the excretion of SN-38-Glu and the carboxylate forms of CPT-11 and SN-38 was greater than that of other non-carboxylate forms of compounds. It is likely that hydrolysis of lactone rings and glucuronidation of SN-38 are the key factors underlying the transition of CPT-11 into the gastrointestinal lumen through hepatocytes.
Maintenance of a high serum level of the lactone form of CPT-11 is important because of the poor antitumor activity of its carboxylate form (16, 17, 21). In this case, biliary excretion of SN-38-Glu is thought to be greater than that of CPT-11 or SN-38. Thus, it is important to inhibit the deconjugation of SN-38-Glu by beta-glucuronidase (4). It has been reported that oral alkalization is useful in preventing CPT-11-induced diarrhea (10). Sodium bicarbonate inhibits absorption of SN-38 by alkalization of the small intestine and has been used to prevent diarrhea. However, high-dose oral administration of sodium bicarbonate could also induce alkalization of blood and subsequently convert lactone forms of these compounds to carboxylate forms due to the fact that the rate of hydrolysis of CPT-11 and its metabolites is greatly increased between pH 7.0 and pH 8.0 (20). It is possible that only a small change in blood pH affects the antitumor activities and pharmacokinetics of CPT-11. Unlike sodium bicarbonate, magnesium oxide is absorbed in the stomach and directly enters the intestinal lumen. Magnesium oxide therefore has little effect on blood pH. It is possible that oral administration of magnesium oxide is more useful than sodium bicarbonate in preventing the occurrence of severe delayed diarrhea induced by CPT-11.
In summary, our results suggest that the rate of conversion of lactone to carboxylate forms of CPT-11 and its metabolites plays a major role in the biliary excretion of these compounds. Our results are considered important data to call for the reasonable use of CPT-11 in clinical practice.
Acknowledgements
We thank Dr. S. Miyauchi for his advice on the experimental technique. This work was supported in part by a grant from the Japan Research Foundation for Clinical Pharmacology.
References
Houghton, P. J., Cheshire, P. J., Hallman, J. C., Bissery, M. S., Mathieru-Boue(e'), A., and Houghton, J. A., Therapeutic efficacy of the topoisomerase I inhibitor 7-ethyl-10-(4-[1-piperidino]-1-piperidino)-carbonyloxy-camptothecin against human tumor xenografts: lack of cross-resistance in vivo in tumors with acquired resistance to the topoisomerase I inhibitor 9-dimethylaminomethyl-10-hydroxycamptothecin. Cancer Res, 53:2823-2829, 1993.
Masuda, N., Fukuoka, M., Kudoh, S., Kusunoki, Y., Matsui, K., Nakazawa, K., Hirashima, T., Tamanoi, M., Nitta, T., Yana, T., Negoro, S., Takifuji, N. and Takada, M., Phase I study of irinotecan and cisplatin with granulocyte colony-stimulating factor support for advanced non-small-cell lung cancer. J Clin Oncol, 12:90-96, 1994.
Rivory, L. P., Bowles, M. R., Robert, J. and Pond, S. M., Conversion of irinotecan (CPT-11) to its active metabolite, 7-ethyl-10-hydroxycamptothecin (SN-38), by human liver carboxylesterase. Biochemical Pharmacol, 52:1103–1111, 1996.
Kehrer, D. F. S., Sparreboom, A., Verweij, J., debrujn, P., Nierop, C. A., Van de Schraaf, J., Ruijgrok, E, J. and De Jonge, M. J. A., Modulation of Irinotecan-induced Diarrhea by Cotreatment with Neomycin in Cancer Patients. Clin Cance Res, 7:1136-1141, 2001.
Abigerges, D., Armand, J. P., Chabot, G. G., Da Costa, L., Fadel, E., Cote, C., Herait, P., and Gandia, D. Irinotecan (CPT-11) high-dose escalation using intensive high-dose loperamide to control diarrhea. J Natl Cancer Inst, 86:446–449, 1994.
Lokiec, F., Canal, P., Gay, C., Chatelut, E., Armand, J. P., Roche, H., Bigat, R., Goncalves, E. and Mathieu-Boue, A., Pharmacokinetics of irinotecan and its metabolites in human blood, bile, and urine. Cancer Chemother Pharmacol, 36:79-82, 1995.
Gupta, E., Lestingi, T. M., Mick, R., Ramirez, J., Vokes, E. E. and Ratain, M. J., Metabolic fate of irinotecan in humans: correlation of glucuronidation with diarrhea. Cancer Res, 54:3723–3725, 1994.
Takasuna, K., Hagiwara, T., Hirohashi, M., Nomura, M., Nagai, E., Yokoiu, T. and Kamataki, T., Involvement of b-glucuronidase in intestinal microflora in the intestinal toxicity of the antitumor camptothecin derivative irinotecan hydrochloride (CPT-11) in rats. Cancer Res, 56:3752–3757, 1996.
Iyer, L., King, C. D., Whitington, P. F., Green, M. D., Roy, S. K., Tephly, T. R., Coffman, B. L.,and Ratain, M. J., Genetic predisposition to the metabolism of irinotecan (CPT-11). Role of uridine diphosphate glucuronosyltransferase isoform 1A1 in the glucuronidation of its active metabolite (SN-38) in human liver microsomes. J Clin Invest, 101:847-854, 1998.
Takeda, T., Kobayashi, K., Akiyama, Y., Soma, T., Honda, S. and Kudo, K., Prevention of irinotecan (CPT-11)-induced diarrhea by oral alkalization combined with control of defecation in cancer patients. Int J Canver, 92:269-275, 2001.
Chu, X. Y., Kato, Y. and Sugiyama, Y., Possible involvement of P-glycoprotein in biliary excretion of CPT-11 in rats. Drug Metab Dispos, 27:440-441, 1999.
Elbling, L., Berger, W., Weiss, R. M., Printz, D., Fritsch, G. and Micksche, M., A novel bioassay for P-glycoprotein functionality using cytochalasin D. Cytometry , 31:187-198, 1998.
Leier, I., Eisenbeiss, J. H., Cui, Y. and Keppler, D., ATP-dependent para-aminohippurate transport by apical multidrug resistance protein MRP2. Kidney Int, 57:1636-1642, 2000.
Chu, X. Y., Kato, Y. and Sugiyama, Y., Multiplicity of biliary excretion mechanisms for irinotecan, CPT-11, and its metabolites in rats. Cancer Res, 57:1934-1938, 1997.
Itoh, T., Takemoto, I., Hata, Y., Takaoka, K., Sugawara, M., Iseki, K. and Miyazaki, K., Determination of the lactone forms and carboxylate forms of Irinotecan and its active metabolite, glucuronide by high-performance liquid chromatography. Jpn J Ther Drug Monit, 17:383-389, 2000.
Rivory, L. P., Chatelut, E., Canal, P., Mathieu-Boue, A. and Robert, J., Kinetics of the in vivo interconversion of the carboxylate and lactone forms of irinotecan (CPT-11) and of its metabolite SN-38 in patients. Cancer Res, 54:6330–6333, 1994.
Rowinsky, E. K., Grochow, L. B., Ettinger, D. S., Sartorius, S. E., Lubejko, B. G., Chen, T. L., Rock, M. K. and Donehower, R. C., Phase I and pharmacological study of the novel topoisomerase I inhibitor 7-ethyl-10-[4-(1-piperidino)-1-piperidino]carbonyl- oxy-camptothecin (CPT-11) administered as a ninety-minute infusion every 3 weeks. Cancer Res, 54:427–436, 1994.
Arimori, K., Kuroki, N., Kumamoto, A., Tanoue, N., Nakano, M., Kumazawa, E., Tohgo, A. and Kikuchi, M., Excretion into gastrointestinal tract of irinotecan lactone and carboxylate forms and their pharmacodynamics in rodents. Pharm Res, 18:814-822, 2001.
Chu, X. Y., Kato, Y., Ueda, K., Suzuki, H., Niinuma, K., Tyson, C. A., Weizer, V., Dabbs, J. E., Froehlich, R., Green, C. E. and Sugiyama, Y., Biliary excretion mechanism of CPT-11 and its metabolites in humans: involvement of primary active transporters. Cancer Res, 58:5137-5143, 1998.
Akimoto, K., Kawai, A. and Ohya, K., Kinetic studies of the hydrolysis and lactonization of camptothecin and its derivatives, CPT-11 and SN-38, in aqueous solution. Chem Pharm Bull, 42:2135– 2138, 1994.
Wani, M. C., Ronman, P. E., Lindley, J. T. and Wall, M. E., Plant antitumor agents. 18. Synthesis and biological activity of camptothecin analogues. J Med Chem, 23:554–560, 1980.
Corresponding Author: Ken Iseki, Department of Clinical Pharmaceutics & Therapeutics, Graduate School of Pharmaceutical Sciences, Hokkaido University, Kita-12-jo, Nishi-6-chone, Kita-ku, Sapporo 060-0812, Japan. ken-i@pharm.hokudai.ac.jp
Published by the Canadian Society for Pharmaceutical Sciences.
Copyright © 1998 by the Canadian Society for Pharmaceutical Sciences.
http://www.ualberta.ca/~csps