J Pharm Pharmaceut Sci (www.ualberta.ca/~csps) 7(1):55-64, 2004
Assessment of enzymatic cleaning agents and disinfectants against bacterial biofilms.
Mona Augustin, Terhi Ali-Vehmas
Department of Basic Veterinary Sciences, Section of Veterinary Microbiology, Faculty of Veterinary Medicine, University of Helsinki, FinlandFaik Atroshi1
Department of Clinical Medicine, Pharmacology & Toxicology, Faculty of Veterinary Medicine, University of Helsinki, FinlandReceived 22 November 2003, Revised 13 January 2004, Accepted 10 February 2004, Published 18 February 2004
PDF Version
Abstract
PURPOSE: Microbial biofilm has become difficult to control by antibiotic and biocide regimes that are effective against suspended bacteria. Their colonization of surfaces can be a problem and is generally controlled through cleaning and disinfection. This study was undertaken to examine the efficacy of the disinfectants including Bio-Ow, Econase CE, Gamanase GC 140, IndiAge 44L, Mannanase AMB, Multifect P-3000, Neutrase, Pandion, Paradigm, Pectinex Ultra SP-L, Promozyme, Resinase A2X, Spezyme AA300, Spezyme GA300 and Vinozym EC, and the proteinase against bacterial biofilms. METHODS: The effectiveness of 20 commercial disinfectants against Pseudomonas aeruginosa (P. aeruginosa) biofilms using a fluorometric technique was examined. Additionally the disinfectants were also tested against Lactobacillus bulgaricus (L. bulgaricus), Lactobacillus lactis (L. lactis) and Streptococcus thermophilus (S. thermophilus) isolates using microtitration tray based turbidimetric techniques. Escherichia coli (E. coli) was used as the test bacteria in the fluorometric control method. RESULTS: Among the first group of the enzymatic cleaning agents tested, four disinfectants (Pandion, Resinase A2X, Spezyme GA300 and Paradigm) were the most potent against bacterial biofilms after 30 min incubation time (residual bacterial count less than 103 CFU (colony forming units)/ml). However, only Resinase A2X and Paradigm showed a good effect on bacterial biofilms after 15 min incubation time. Proteinase disinfectants (alkalase, chymotrypsin, cryotin and krilltrypsin) from the second group of the disinfectants showed a good effect against P. aeruginosa biofilm when tested in the absence of milk. The performance of the disinfectants was reduced in the presence of milk. The minimum inhibitory concentration (MIC) of the cleaning agents was determined as the lowest concentration inhibiting bacterial growth. The MIC was tested on Lactobacillus bulgaricus (L. bulgaricus), Lactobacillus lactis (L. lactis) and Streptococcus thermophilus (S. thermophilus) isolates. The minimum inhibitory concentrations (MIC) for Paradigm against S. thermophilus and L. Lactis were lower than L. Bulgaricus. Whereas, the MIC of Pandion against L. bulgaricus was lower than MIC against L. lactis. Resinase A2X had no inhibitory effect on bacterial growth when the concentration was less than or equal to 2.4 mg/ml and Spezyme GA 300 concentration less than or equal to 7.3 mg/ml. Minimum inhibitory concentration of Pandion against L. bulgaricus was 2.7 mg/ml and against L. lactis 5.3 mg/ml. Growth of S. thermophilus was inhibited in all concentration of Pandion tested. CONCLUSIONS: The choice of disinfectant or cleaning agent along with the optimum concentration and the time of action is very important when destroying microbes. It is also important that the resistances of microbes to different disinfectants and cleaning agents be taken into account when planning the cleaning process.
Introduction
The development of pharmaceuticals for the antimicrobial agents including disinfectants is well known. However, increasing problems associated with the use of these agents' calls for the development of ideal disinfectants, which would act quickly and not produce unknown byproduct remains the primary challenge to approach. Disinfection is a process where pathogenic microorganisms are removed or inactivated by chemical (e.g., chlorination) or physical (e.g., filtration, UV radiation) means such that they represent no significant risk of infection (1). An ideal disinfectant should have a broad spectrum of antimicrobial activity, but it should be nontoxic and nonirritating (1). It should also be compatible with the surfaces to be disinfected, easy to prepare and use, stable, affordable, and readily available, and it should lack any unpleasant odor. However, no antiseptic or disinfectant is ideal, each falling short of meeting the aforementioned criteria. Mandell et al (2) defined three different categories of disinfectants:(a) high-level disinfectants that are effective against viable endospores if the exposure is long enough; (b) intermediate-level disinfectants that are tuberculocidal but not sporicidal; (c) low-level disinfectants that do not reliably destroy bacterial endospores, tuberculous bacilli, or small, non lipid RNA viruses (e.g. enterovirus). Consequently, efforts are continuously under way to improve the performance of existing disinfectants or develop new ones. The efficacy of antimicrobial active ingredients may be greatly affected by the formulation of the product, such that products with the same levels of antimicrobial may demonstrate varying levels of effectiveness. Efficacy may be affected by pH, detergent base, emollients and humectants, ionic nature of the formulation, and type of surfactants.
Bacteria are amongst the most successful living organisms and their ubiquity ensures that humans are obliged to live in constant and intimate contact with a wide variety of species. Although the number of species capable of causing disease is relatively few, many others have the ability to do so given the right conditions (3). During the last 10-15 years, antibiotic resistance has increased (4). Over use of antibiotics in humans has led to the rapid evolution of bacteria that are resistant to multiple drugs. Apart from the discovery and exploitation of the natural peptide antimicrobial agents that form part of the innate immune systems of plants and animals, there have been few new antibiotics developed in recent years (5). In addition, some bacteria have resistance to biocides such as antiseptics, disinfectants and preservatives, which poses a potential challenge for future bacteria control (6). With this in mind, there will be a continued requirement for new and potent antimicrobial agents, together with techniques suitable for the control and destruction of bacteria.
Biofilms are composed of populations of microorganisms in an extra cellular matrix adhering to surfaces in which sufficient moisture is available. They are present in living organisms in addition to the surfaces of inanimate objects (7, 8, 9, 10, 11, and 12). Biofilm formation causes problems in many areas, e.g. in industrial water systems, in medicine and in food processing industry (13, 14, 15,16,17,18). There are serious health and medically related problems caused because of microbial biofilm growth. Bacteria growing as surface associated biofilms are much harder to treat with anti-microbials thus potentially leading to the build up dangerous infections. The growth patterns, coverage and adherence of the biofilms depend critically on the substrate roughness, composition, type of microorganisms and other factors (17, 18, 19, 20, 21, and 22).
Several reasons can account for the reduced sensitivity of bacteria within a biofilm. There may be (i) reduced access of a disinfectant to the cells within the biofilm, (ii) chemical interaction between the disinfectant and the biofilm itself, (iii) modulation of the microenvironment, (iv) production of degradative enzymes (and neutralizing chemicals), or (v) genetic exchange between cells in a biofilm. However, bacteria removed from a biofilm and recultured in liquid culture media are generally no more resistant than the "ordinary" planktonic cells of that species (25). Milk is a good growth medium for many microorganisms because of its high water content, neutral pH, and variety of available nutrients. Dairy products provide substantially different growth environments than fluid milk because these products have nutrients removed or concentrated or have lower pH water activity (26).
Enzymes can be used for degradation of biofilm but due to the heterogeneity of the extracellular polysaccharides in the biofilm, a mixture of enzyme activities may be necessary for a sufficient degradation of a bacterial biofilm. The application of enzymes for control of protein biofilm on surfaces and in closed pipelines is well known. In particular, proteases are used in pipelines and for removal of protein from contact lenses. The use of enzymes for removal of bacterial biofilm is still limited, partly due to the very low prices of chemicals in use. In addition, the lack of techniques for quantitative evaluation of the effect of enzymes, as well as the commercial accessibility of different enzyme activities, limits their usage. It is known that monocomponent enzymes can be used for biofilm removal. However, the heterogeneity of the biofilm matrix limits the potential of monocompound enzymes (27).
The activity of disinfectants in vitro are currently tested either on microorganisms in suspension or on germ carriers. The mix microorganisms in suspension with a solution of the compound under test, in the presence or absence of interfering substances selected as a function of the field of application of the agent and the mode of use seems the most common method (28, 29,30,31,32,33). However, marked discrepancies between these methods are not uncommon. Therefore, it is necessary to perform and develop a rapid and predictive tool for in vitro antimicrobial studies of the efficacy of the disinfectants. No information is available regarding the efficacy of the disinfectants studied including Bio-Ow, Econase CE, Gamanase GC 140, IndiAge 44L, Mannanase AMB, Multifect P-3000, Neutrase, Pandion, Paradigm, Pectinex Ultra SP-L, Promozyme, Resinase A2X, Spezyme AA300, Spezyme GA300 and Vinozym EC, and the proteinase disinfectants against bacterial biofilms. Furthermore, we studied the sensitivity of microtitration tray based fluorometric assay in analyzing enzymatic activity of P. aeruginosa and E. coli and finally specified the effect of disinfectants against biofilms.
Materials and Methods
Disinfectants. The disinfectants (enzymatic cleaning agents) used in this study were tested. Enzymes were originated from two sources. First group were originated from VTT Biotechnology and Food Research Institute (Espoo, Finland). They include Bio-Ow, Econase CE, Gamanase GC 140, IndiAge 44L, Mannanase AMB, Multifect P-3000, Neutrase, Pandion, Paradigm, Pectinex Ultra SP-L, Promozyme, Resinase A2X, Spezyme AA300, Spezyme GA300 and Vinozym EC. The second group was proteinase agents, they were originated from University of Iceland: Cryotin and Chymotrypsin were from cod, alkalase was from Novo, Krilltrypsin was from Antarctic krill. The proteinase samples were isolated and prepared in Iceland.
Microorganisms. Escherichia coli (PT 238), Pseudomonas aeruginosa (ATCC-215442), Lactobacillus lactis (VTT E-85 222), Streptococcus thermophilus (VTT E-96 665) and Lactobacillus bulgaricus (VTT E-96662) were used as test organisms.
Media. The broth media used in the study were ISB (Iso-Sensitest Broth, Unipath ltd., Basingstoke, Hampshire, England) and M.R.S (M.R.S Broth,Unipath ltd., Basingstoke, Hampshire, England). The media were prepared according to the manufacturer's instructions.
Fluorogenic substrates . The fluorogenic substrates used were 4-methylumbelliferyl- β -D-glucuronide (4-MUG) (Sigma Inc., St Louis, MO, USA), 4-methylumbelliferyl phosphate-Li 2 (4-MUP), (Sigma Inc., St Louis, MO, USA) and resazurin (Sigma, R-217, St Louis, MO, USA).
Major equipment . Fluorometric studies of bacterial enzymatic activity were carried out using microtitration-tray reading fluorometer Fluoroskan 2 (Labsystems, Helsinki, Finland). For washing procedures, the Labsystem's Wellwash 4 microplate ELISA washer (Labsystems, Helsinki, Finland) was used. Water bath was used for incubation. Turbidimetric studies of bacterial growth were carried out using the Bioscreen® analyzer (Labsystems, Helsinki, Finland), a fully automated analyzing system for measuring bacterial growth with a vertical light path, 200 individual tests could be run simultaneously.
Preparation of biofilms. 50 ml of milk was allowed to dry three days on surfaces of the test wells of microtitration trays by evaporation in a laminar. After drying, the plates were covered with plastic coat and stored in cold (4░C). P. aeruginosa were allowed to grow and develop biofilm for 1 day (+37░C) in wells using ISB as the growth medium. This method produced biofilm with about 107 CFU/ml.
Washing procedure and tests on cleaning agents. After incubation of P. aeruginosa , the wells were rinsed twice with sterile water and then washed once with ELISA washer before enzyme treatment. A dilution series of enzymes (100%-1.5% enzyme product) was prepared in sterile water. The enzyme dilutions were added to wells of a microtitration tray (200 m l to each well, 8 repeats). The tray containing the enzyme dilutions was incubated 30 min and 15 min at optimal conditions for each enzyme (pH and temperature). After incubation, the enzyme product was removed from the wells and washed three times with sterile water. Medium and the flurogenic substrate were added to each well before the measurement. With proteinase samples, two different concentrations were used in final assay system. Sample dilutions were buffered at pH 8 and temperature used was 5░C. Duration of incubation was 2 h and 24 h.
Fluorometric analysis of bacterial phosphatase activity. The bacteria used was P. aeruginosa (ATCC - 2 15442). The bacteria were harvested from blood-agar plates, inoculated into ISB-broth and allowed to grow overnight at 37░C. A tenfold dilution series was prepared in the 0.9% NaCl. The initial bacterial numbers in the wells of the microtitration trays corresponded to 103 -107 CFU/ml. A stock solution of 4-MUP (4-Methylumbelliferyl phosphate-Li2) was prepared freshly by dissolving 4-MUP (1mg/ml) in saline and filter-sterilized (34). The final concentration of 4-MUP in sample mixtures was 0.2 mM. The bacterial suspension (50 ml, initial concentration 103 -107 CFU/ml in wells, 8 replicates) was dispensed into microtitration tray wells containing 50 ml dry milk, 30 ml 4-MUP and 220 ml ISB. The release of 4-MU over time by bacterial phosphatase was automatically monitored at 37░C for 12 hour (excitation 355 nm and emission 460 nm).
Fluorometric analysis of bacterial b-glucuronidase activity. The bacteria strain used was E.coli PT-238. The bacteria were harvested from blood-agar plates, inoculated into ISB-broth and allowed to grow overnight at 37°C. A tenfold dilution series was prepared in the 0.9% NaCl. Therefore, the initial bacterial numbers in the wells of the microtitration trays corresponded to 103 -107 CFU/ml. A stock solution of 2 mM [4-MUG (4- Methylumbelliferyl- b -D-glucuronide)] was prepared freshly by dissolving 7 mg 4-MUG in ISB and filter-sterilized (35). A final concentration of 4-MUG in sample mixtures was 0.2 mM. The bacterial suspension (50 ml, initial concentration 10 3 -107 CFU/ml in wells, 8 replicates) was dispensed into microtitration tray wells containing 50 ml dry milk, 30 ml MUG-solution and 220 ml ISB. The release of 4-MU (4- Methylumbelliferone) over time by bacterial b-glucuronidase was automatically monitored at 37°C for 12 hour (excitation 355 nm and emission 460 nm).
Bacterial growth studies using resazurin as a fluorogenic substrate. The bacteria used was P. aeruginosa (ATCC - 2 15442). Bacteria were harvested from blood-agar plates, inoculated into ISB-broth and allowed to grow overnight at 37°C. A ten fold dilution series was prepared in the 0.9% NaCl. The initial bacterial numbers in the wells of the microtitration trays corresponded to 103 -107 CFU/ml. A stock solution of resazurin (0.05 mg/ml) was prepared by dissolving resazurin in sterile water. The final concentration of resazurin in sample mixtures was 0.006 mg/ml. The bacterial suspension (50 m l, initial concentration 107 CFU/ml in wells, 8 replicates) was dispensed into microtitration tray wells containing 50 m l dry milk, 20 m l resazurin and 230 ml ISB. P. aeruginosa were allowed to grow for one day. The fluorogenic resazurin reaction for bacterial activities was monitored 12 hour at 37°C (excitation 544 nm and emission 584 nm).
Analysis of the minimum inhibitory concentration (MIC) . Bacteria were harvested from MRS-plates and allowed to grow overnight at 37°C. To specify the minimum inhibitory concentration (MIC), turbidometric bacterial growth studies (36) were carried out, using the Bioscreen analyzer (Labsystems). L. bulgaricus, L. lactis and S. thermophilus were used as test strains. Bacterial suspension (30 m l initial concentration 106 -10 7 CFU/ml in wells) was dispensed into honeycomb wells containing 240 ìl MRS-broth and 30 m l enzyme dilution. The honeycomb plates were covered and pre-incubated for 10 min. The change in turbidity was monitored automatically every 20 minutes for 24 hour at 37°C using wide-band filter (wide band, absorbance at 420, 450, 492, 540, and 580 nm). The minimum inhibitory concentrations of the cleaning agents were determined as the lowest concentration inhibiting bacterial growth.
Statistical analysis . Data was expressed as the mean ± standard error of the mean (SEM). Statistical procedures were performed using linear regression analysis. Statistical significance was defined as P < 0.05.
Results
Determination of the disinfectants effect on bacterial cells . The resazurin-based fluorometric method found to be more promising to evaluate the functional status of disinfectants than 4-MUP ( P. aeruginosa ) and 4-MUG (E. coli)-based methods giving a combination of greater fluorescence over a broad pH range and reduced growth inhibition with P. aeruginosa biofilms. The resazurin-based fluoromety method generated a more rapid fluorescence than any other tested substrate.
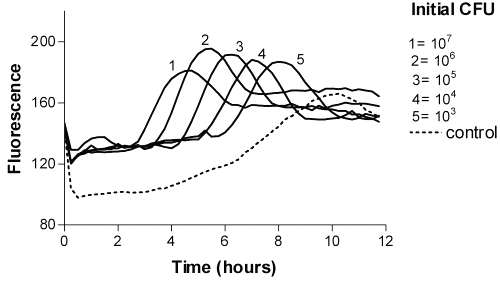
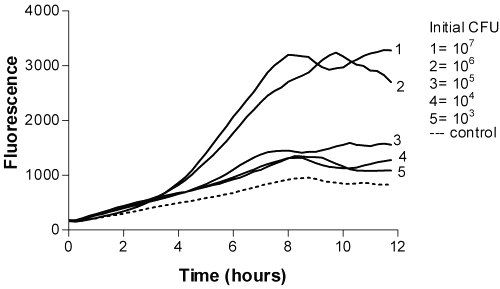
Even with the smallest E. coli inoculum in milk, CFU more than 103 with 4-MUG (Figure 1) and 106 with P. aeruginosa 4-MUP (Figure 2) could be quantified within 8 hours of incubation.
Figure 1: Time-fluorescence curves of E. coli 4-MUG as a fluorogenic substrate.
Figure 2: Time-fluorescence curves of P. aeruginosa 4-MUP as a fluorogenic substrate.
The fluorescence of 4-MU did not reach the maximum value due to the decreasing pH caused by bacteria. However, 4-MU is more fluorigenic at alkalic conditions. The timing of the appearance of maximum fluorescence of resazurin was directly related to the logarithm of the number of colony-forming units (Figures 3 and 4).
Figure 3: Time-fluorescence curves of P. aeruginosa Resazurin as a fluorogenic substrate.
Figure 4: Time-fluorescence curves of P. aeruginosa Resazurin as a fluorogenic substrate (in ISB).
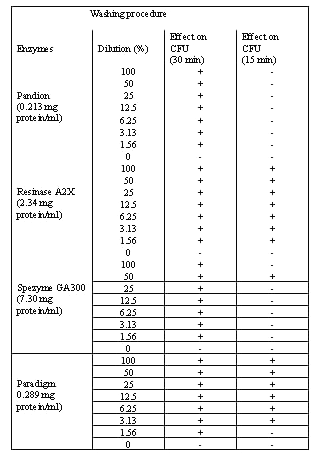
Cleaning agents and proteinases . Four of the cleaning agents (Pandion, Resinase A2X, Spezyme GA300 and Paradigm) had a significant effect against bacterial biofilms after 30 min incubation time using the resazurin method (Table 1).
Table 1: Effect of enzymes treatment on P. aeruginosa biofilm. Bacterial concentrations were interpolated from the time-fluoresence response curves of P. aeruginosa (CFU/ml). Bacterial biofilms on microtitration trays were incubated over 15 and 30 minuets at optimal temperatures and pH before measurement. Enzymes concentrations are indicated in brackets.
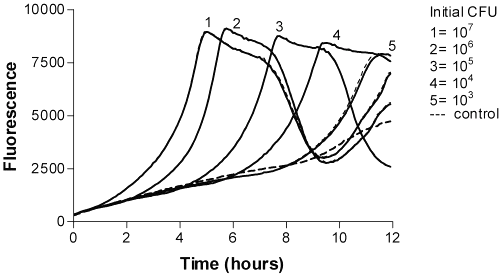
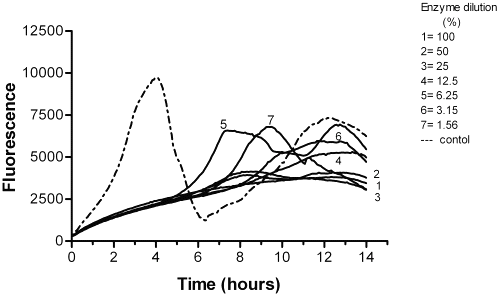
In Pandion and Spezyme GA300, the dropped in the residual bacterial counts were from 107 to 103 CFU/ml (Figure 5).
Figure 5: The effect of Pandion on P. aeruginosa biofilm after 30 min incubation.
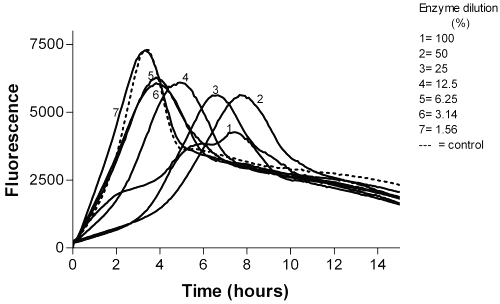
Whereas, Resinase A2X and Paradigm showed a reduction effect from 106 to 103 CFU/ml against bacterial biofilms after 15 min exposure (Table 1 and Figure 6).
Figure 6: The effect of Paradigm on P. aeruginosa biofilm after 15 min incubation.
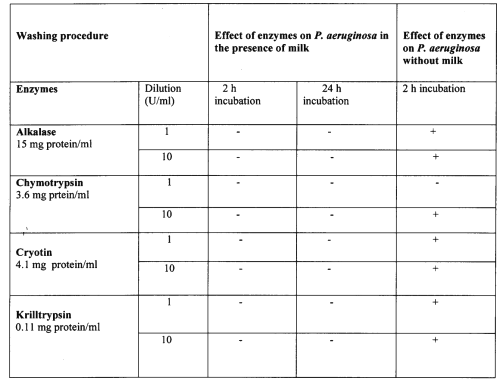
When proteinase agents were considered, a significant effect against Pseudomonas biofilm was seen when milk was not included in the suspension. However, the proteinase agents had no effect on biofilm in the present of milk (Table 2).
Table 2: Effect of proteinase agents on P. aeruginosa biofilms.
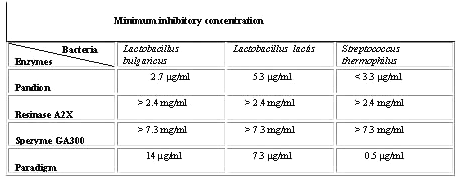
Minimum inhibitory concentration (MIC). The MIC of the enzymatic cleaning agents is given in Table 3.
Table 3: The minimum inhibitory concentration (MIC) for the most effective cleaning agents on Lactobacillus bulgaricus, Lactobacillus lactis and Streptococcus thermophilus.
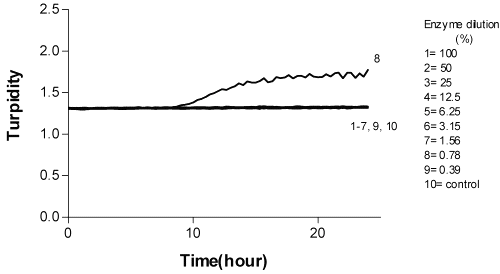
The MIC of the cleaning agents was determined as the lowest concentration inhibiting bacterial growth. Resinase A2X and Spezyme GA 300 had no inhibitory effect on bacterial growth when Resinase A2X and Spezyme concentrations were less than or equal to 2.4 mg/ml and 7.3 mg/ml simultaneously. Minimum inhibitory concentration of Pandion against Lactobacillus bulgaricus and Lactobacillus lactis were 2.7 mg/ml and 5.3 m g/ml simultaneously. Streptococcus thermophilus growth was inhibited by all concentrations of Pandion tested. Minimum inhibitory effect of Paradigm against Lactobacillus bulgaricus was 14 m g/ml, against Lactobacillus lactis was 7.3 mg/ml and against Streptococcus thermophilus was 0.5 m g/ml (Table 3 and Figure 7).
Figure 7: The effect of Pandion on Streptococcus thermophilus E-96 665.
Discussion
In this study, we have demonstrated the rapid microbicidal action of the disinfectants Pandion, Resinase A2X, Spezyme GA300 and Paradigm on P. aeruginosa biofilms. Although Pandion and Spezyme GA300 demonstrated, potent activity against strains tested at initial cell densities as high as 107 CFU/ml, the microbicidal effect decreased with increasing dilution of the disinfectants. However, when the initial cell density was reduced to 103 CFU/ml, 100% killing of the test strain was achieved with only 10% of these enzymes.
In contrast to many other chemical disinfectants, which may require high concentrations and several minutes of exposure (37), the lethal microbicidal effects of the cleaning enzymes of the present study occurred within the first minute of exposure. The absence of significant microbicidal activity after 1 minute of exposure suggests that enzymes used might be very unstable. However, preliminary data (not shown) demonstrate that Pandion remains active in both undiluted and diluted forms for at least 24 hours after preparation. These results suggest that the loss of microbicidal activity after 1 minute of exposure is associated with the presence of the microorganisms rather than the innate instability of Pandion. There are two possible explanations for this mechanism; the first one is the possibility of slow or incomplete penetration of the disinfectants into the biofilm. For example, measurements of antibiotic penetration into biofilms in vitro have shown that some antibiotics readily permeate bacterial biofilms (38). The second explanation depends on an altered chemical microenvironment within the biofilm. Microscale gradients in nutrient concentrations are a well known feature of biofilms. Findings from studies with miniature electrodes have shown that oxygen can be completely consumed in the surface layers of a biofilm, leading to anaerobic niches in the deep layers of the biofilm (39). Concentration gradients in metabolic products mirror those of the substrates. Local accumulation of acidic waste products might lead to pH differences greater than 1 between the bulk fluid and the biofilm interior (40) which could directly antagonise the action of an antibiotic.
The results of the present study show that individual products may show variations in efficacy against microbial biofilms. Paradigm and Resinase A2X were the most potent disinfectants at low concentrations and short test period against P. aeruginosa biofilm (residual bacterial count less than 103 CFU/ml). Paradigm and Resinase A2X may be useful for inactivating of biofilms produced in milk and in other lipid food residues that may remain after poor cleaning in many industrial types of equipment.
When proteinase disinfectants were considered, the results of this study show that these disinfectants were effective against P. aeruginosa biofilms when milk was not added directly to the proteinase solution. The elimination of biofilm is a very difficult and demanding task because many different factors affect the detachment. It is known that monocomponent enzymes can be used for biofilm removal. However, the heterogeneity of the biofilm matrix limits the potential of these enzymes. Our findings suggest that numerous factors play a role in the resistance of biofilm-embedded bacteria. Any of the following alone or in combination, may contribute to compromised antibacterial activity: interaction between constituents of the exopolymer matrices and the antimicrobial agent's suppression of growth rate due to a modified nutrient environment, and dense bacterial inocula. Hostacka (41) studied the effect of commercially manufactured disinfectants against P. aeruginosa and found that the disinfectants tested depressed Pseudomonas aeruginosa virulence factors (elastase and proteinase). The degree of inhibition was directly proportional to the disinfectant concentration.
Since the duration of treatment is one of the important factors affecting the antimicrobial activity of disinfectants, it is desirable to use a sufficiently long cleaning procedure in the food industry. The results after 15 min exposure times showed high activity against P. aeruginosa. According to the provisional European and national surface test methods, 60 min disinfection may be suitable as a disinfection time (42, 29). However, in the food industry there is a trend towards longer production runs with shorter intervals for disinfection and cleaning (43). On the other hand, not only the contact time but also the concentration and the composition of the agent affect the antimicrobial activity. In this study four of the cleaning agents tested (Pandion, Resinase A2X, Spezyme GA300 and Paradigm) had a significant effect (residual bacterial count less than 10 3 CFU/ml) against bacterial biofilms in 30 min. Accordingly it is important to use higher concentrations of the cleaning and disinfection agents, especially in the presence of organic materials. Considerable variation in resistance has been observed between the different species of microbes. It has been demonstrated that gram-negative bacteria biofilms are more resistance than other species. On the other hand, spores have several strong layers, which make it difficult for disinfectants to penetrate them (44, 45). Therefore, it is necessary to use a longer disinfection time and a stronger concentration of disinfectants to destroy biofilms. These findings show the importance of correct application of disinfectants for both concentration and exposure time in the dairies.
Another interesting point in this study was the methods used to study the effect of the disinfectants against bacterial biofilms. We have shown that the susceptibility of bacterial biofilms to the disinfectants tested can be assessed by resazurin based fluorometry. The resazurin based fluorometric method used in this study seems to be more sensitive and reliable than 4-MUP or 4-MUG based fluorometry. 4-MU was more fluorescent at alakaline pH. Therefore, with resazurin based fluorometric method it was possible to define the most effective disinfectants.
Conclusion
Bacteria that adhere to implanted food plants, medical devices or damaged tissue can become the cause of persistent infections (39, 41, and 42). These bacteria encase themselves in a hydrated matrix of polysaccharide and protein, forming a slimy layer known as a biofilm. The mechanisms of resistance to disinfectants in bacterial biofilms can be explained by an altered chemical microenvironment. Microscale gradients in nutrient concentrations are a well known feature of biofilms. Earlier studies with miniature electrodes have shown that oxygen can be completely consumed in the surface layers of a biofilm, leading to anaerobic niches in the deep layers of the biofilm (44). The other possible mechanism is that a subpopulation of micro-organisms in a biofilm forms a unique, and highly protected, phenotypic state--a cell differentiation similar to spore formation. This explanation is lent support by findings from studies that show resistance in newly formed biofilms, even though they are too thin to pose a barrier to the penetration of either an antimicrobial agent or metabolic substrates (45). Nevertheless, the hypothesis of a spore-like state entered into by some of the bacteria in a biofilm provides a powerful, and generic, explanation for the reduced susceptibility of biofilms to antibiotics and disinfectants of widely different chemistries (46). Our results demonstrate that the choice of disinfectant or cleaning agent along with the optimum concentration and the time of action is very important when destroying microbes. It is also important that the resistance of microbes to different disinfectants and cleaning agents be taken into account when planning the cleaning process. Another out come of this study is that the microtitration tray based fluorometric assay provided high throughput capacity system of analyzing various enzymatic cleaning agents for biofilms formed by bactaria.
References
McDonnell G, and Russell D. Antiseptic and Disinfectants: Activity, action, and resistance. Clin Microbiol Rev 1999; 12:147-179.
Mandell GL, Bennet JE y, Dolin R., Principles and Practice of Infectious Diseases. New York, Churchill Livingstone, pp.199-212, 1995.
Schorer M, Eisele M. Accumulation of inorganic and organic pollutants by biofilms in the aquatic environment. Water, Air, Soil Pollut 1997; 99:651–659.
Percival SL, Knapp JS, Edyvean R, Wales DS. Biofilm development on stainless in mains water. Water Res 1998; 32:243–253.
Nielsen PH, Jahn A, Palmgren R. Conceptual model for production and composition of exopolymers in biofilms. Water Sci Technol 1997;36:11–19.
Fuchs S, Haritopoulou T, Wilhelmi M. Biofilms in freshwater ecosystems and their use as a pollutant monitor. Water Sci Technol 1996; 34:137–140.
Drexler KE. Molecular nanomachines: physical principles and implementation strategies. Ann Rev Biophys & Biomol Structure 1994; 23:377-405.
Stoffler D, Steinmetz MO, Aebi U. Imaging biological matter across dimensions: from cells to molecules and atoms. FASEB J 1999; 13 :S195-S200.
Mattila K, Weber A, Salkinoja-Salonen MS. Structure and on-site formation of biofilms in paper machine water flow. J Indust Microbiol & Biotech 2002; 28:268-279.
O’Toole H, Kaplan B, Kolter R. Biofilm formation as microbial development. Ann Rev Microbiol 2000; 54:49–79.
Gilbert P, Allison DG, McBain AJ. Biofilms in vitro and in vivo: do singular mechanisms imply cross-resistance? J Appl Microbiol 2002; 92:S98-S110.
Carpentier B, and Cerf O. Biofilms and their consequences, with particular
references to hygiene in the food industry: a review of the literature. J Appl Bacteriol 1993; 75, 499-511.
Grobe S, Wingender J, Flemming HC. Capability of mucoid Pseudomonas aeruginosa to survive in chlorinated water. Inter J Hygien & Environ Health 2000; 204:139-142.
Wirtanen G. Biofilm formation and its elimination from food processing equipment. Helsinki University of Tecnology. PhD thesis, Espoo, Finland,1995.
Gibson H, Taylor JH, Hall KE, Holah JT. Effectiveness of cleaning techniques used in the food industry in terms of the removal of bacterial biofilms. J Appl Microbiol 1999; 87:41-48.
Moller S, Pedersen AR, Poulsen LK, Arvin E, Molin S. Activity and three-dimensional distribution of toluene-degrading Pseudomonas putida in a multispecies biofilm assessed by quantitative in situ hybridization and scanning confocal laser microscopy. Appl & Environ Microbiol 1996; 62:4632-4640.
Schwartz T. Hoffmann S. Obst, U. Formation of natural biofilms during chlorine dioxide and U.V. disinfection in a public drinking water distribution system. J Appl Microbiol. 2003; 95:591-601.
Codony F, Domenico P, Mas J. Assessment of bismuth thiols and conventional disinfectants on drinking water biofilms. J Appl Microbiol 2003; 95:288-293.
Jayaraman A, Sun AK, Wood TK. Characteriz ation of axenic Pseudomonas fragi and E. coli biofilms that inhibit corrosion of SAE 1018 steel. J Appl Microbiol 1998; 84:485–492.
Ben-Jacob E, Schochet O, Tenenbaum A, Cohen I, Czirok A, Vicsek T. Generic model of co-operative growth patterns in bacterial colonies. Nature 1994; 368:46–49.
Moller S, Korber DR, Wolfhaardt GM, Molin S, Caldwell DE. Impact of nutrient composition on a degradative biofilm community. Appl Environ Microbiol 1997; 63:2432–2438.
Stephens C. Microbiology: breaking down biofilms. Current Biol 2002; 12:R132-R134.
Richter A, Smith R, Ries R, Wildenauer F. Features of biofilm growth on solid surfaces analysed by Monte Carlo simulations. Phys Stat Sol A 1999; 176:953–967.
Hentzer M, Eberl L, Nielsen J. Givskov, M. Quorum Sensing: A Novel Target for the Treatment of Biofilm Infections. Biodrugs. 2003; 17:241-250.
Brown MRW and Gilbert P. Sensitivity of biofilms to antimicro-bial agents. J Appl Bacterio 1993; 74:S87–S97.
Frank, JF., Milk and dairy products, in Food Microbiology, Fundamentals and Frontiers: Doyle MP, Beuchat LR, Montville TJ (eds), ASM Press, Washington. pp 101, 1997.
Johansen C, Falholt P, and Gram L. Enzymatic removal and disinfection of bacterial biofilms Appl Environment Mikrobiol 1997; 63: 3724-3728.
Kelsey JC. Disinfectants and methods of testing. J Med Lab Tech 1969; 26:79-89.
Van Klingeren B. Disinfectants testing on surfaces. J Hosp Infect 1995; 30: S397-S408.
Bloomfield SF, Scott E. Cross-contamination a,d infection in the domestic environment and the role of chemical disinfectants. J Appl Microbiol 1997; 83:1-9.
Ntsama-Essomba C, Bouttier S, Ramaldes M, Dubois-Brissonnet F, Fourniat J. Resistance of Escherichia coli growing as biofilms to disinfectants. Vet Res 1997; 28:352-363.
Langsrud S, Sundheim G, Borgmann-Strahsen R. Intrinsic and acquired resistance to quaternary ammonium compounds in food-related Pseudomonas spp.. J Appl Microbiol 2003; 95:874-882.
Cole EC, Addison RM, Rubino JR, Leese KE, Dulaney PD, Newell MS, Wilkins J, Gaber DJ, Wineinger T, Criger DA. Investigation of antibiotic and antibacterial agent cross-resistance in target bacteria from homes of antibacterial product users and nonusers. J Appl Microbiol 2003; 95:664-676.
Ali-Vehmas T, Rizzo A, Westermarck T, Atroshi F. Measurement of antibacterial activities of T-2 Toxin, deoxynivalenol, ochratoxin A, aflatoxin B1 and fumonisin B1 using microtitration tray-based turbidimetric techniques. J Vet Med A 1998; 45:453-458.
Atroshi F, Ali-Vehmas T, Westermarck T, Rizzo A, Selga G, Baltais A, Linars J, Saulite V, Daukste A. Evaluation of the antibacterial and hemolytic activities of Latvian herbal preparation. Vet & Human Toxicol 2000; 42:341-344.
Ali-Vehmas T, Westphalen P, Myllys V, Sandholm M. Binding of Staphylococcus aureus to milk fat globules increases resistance to penicillin-G. J Dairy Res 1997 64:253-260.
Olmsted, RN.; APIC Infection Control and Applied Epidemiology: Principles and Practice. St Louis. MO: Mosby-Year Book, Inc; pp 34-39, 1996.
Stewart PS. Theoretical aspects of antibiotic diffusion into microbial biofilms. Antimicrob Agents Chemother 1996; 40: 2517–2522.
de Beer D, Stoodley P, Roe F, Lewandowski Z. Effects of biofilm structure on oxygen distribution and mass transport. Biotechnol Bioeng 1994; 43: 1131–1138.
Zhang TC, Bishop PL. Evaluation of substrate and pH effects in a nitrifying biofilm. Wat Environ Res 1996; 68: 1107–1115.
Hostacka A. Influencing virulence factors of Pseudomonas aeruginosa by commercially manufactured disinfectants. Pharmazie 1997; 52:317-319.
Bloomfield SF, Arthur M, Van Klingeren B, Pullen W, Holah JT, Elton R. An evaluation of the repeatability and reproducibility of a surface test for the activity of disinfectants. J Appl Bacteriol 1994; 76:86-94.
Rutala WA. APIC guideline for selection and use of disinfectants. Amer J Infec Control 1996; 24:313-342.
Wirtanen G, Salo S, Allison DG, Mattila-Sandholm T, Gilbert P. Performance evaluation of disinfectant formulations using poloxamer-hydrogel biofilm-constructs. J Appl Microbiol 1998; 85:965-971.
Gilbert P, Allison DG, McBain AJ . Biofilms in vitro and in vivo: do singular mechanisms imply cross-resistance? J Appl Microbiol 2002; 92: 98S-110S.
Corresponding Author: Faik Atroshi, Department of Clinical Medicine, Pharmacology & Toxicology, Faculty of Veterinary MediciČne, P.O. Box 57, FIN-00014 Helsinki University, Finland. faik.atroshi@helsinki.fi
Published by the Canadian Society for Pharmaceutical Sciences.
Copyright ę 1998 by the Canadian Society for Pharmaceutical Sciences.
http://www.ualberta.ca/~csps